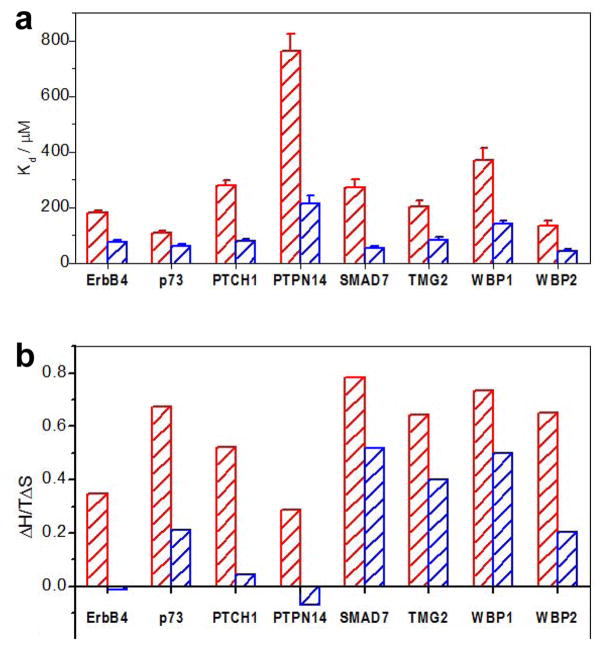
Figure 3.
Comparison of thermodynamic properties for the binding of WW1 domain alone (red) and in the context of WW1-WW2 tandem module (blue) of WWOX to various peptides. (a) Comparison of equilibrium dissociation constant (Kd) associated with binding. Error bars were calculated from at least three independent measurements to one standard deviation. (b) Comparison of TΔS/ΔH ratio, where ΔH and TΔS are the enthalpic and entropic changes associated with binding, respectively.