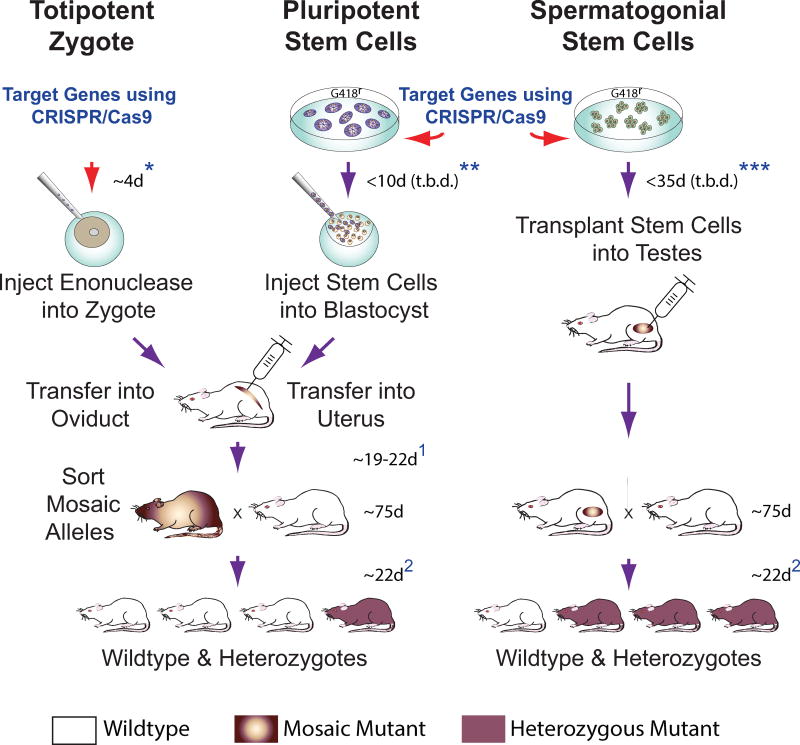
Figure 4. CRISPR/Cas9 Gene Targeting in early Embryos and Spermatogonia.
(Left) Zygotes are injected with CRISPR/Cas9 constructs and transferred into surrogate female oviducts to support donor embryo development into mutant progeny. Mosaic mutant progeny born ~19–22 d post-transfer (depending on cleavage stage transferred)1 display variation in targeted alleles in various tissues, and must be crossed to wildtype stock to generate pure heterozygous mutants isogenic at respective targeted alleles in all cells of their body.
(Center) Rat Pluripotent Stem Cell cultures could prospectively be genetically modified using CRISPR/Cas9. Rat ES cells with targeted mutations have been selected in culture prior to blastocyst injection (Tong et al., 2011). Injected blastocysts are transferred into uteri of surrogate females to produce mosaic/chimeric mutant animals ~19 d post-transfer1. Mosaic/chimeric animals are crossed to wildtype stock to establish pure heterozygous mutants.
(Right) Spermatogonial Stem Cells can be genetically modified in culture using CRISPR/Cas9. Modified spermatogonia are injected into recipient rat testes to produce mutant spermatozoa that transmit targeted genomic modifications to heterozygous mutant progeny. Timelines for each approach listed above must consider ~75 d for rat breeder pairs to reach reproductive age, 21–23 d rat gestation time, plus a 4–5 d estrus cycle in rats (Lohmiller and Swing, 2006)2.
*Includes additional 4 d to establish pseudo-pregnant female recipients by paring with vasectomized males; does not include time needed to prepare vasectomized male rats.
**Remains to be determined (t.b.d) using CRISPR/Cas9; estimate based on rat ES cell lines selected following transfection with classical DNA targeting constructs (Tong et al., 2011); includes additional 4 d to establish pseudo-pregnant female recipients.
***Current study; minimum time required following delivery of CRISPR/CAS9 constructs to spermatogonia (with or without genetic selection) prior to transplantation was not studied and remains to be determined (t.b.d); includes 12 d to prepare recipient males.