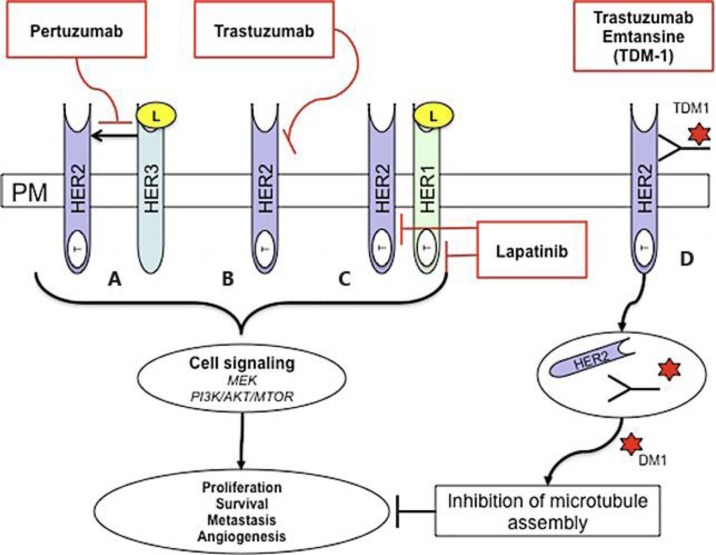
Fig. 1.
HER2-targeted therapies. Human epidermal growth factor (EGF) HER1, HER2, HER3 and HER4 are receptor tyrosine kinases within the plasma membrane (PM) that are involved in signal transduction pathways that modulate cellular processes. They consist of an extracellular ligand binding domain and intracellular tyrosine kinase domain (T) (except for HER3). The ligand (L) binds the receptor extracellular domain, homo-dimerisation and hetro-dimerisation leads to tyrosine kinase domain phosphorylation and activation of downstream signaling pathways. These include the MEK and PI3K/AKT/MTOR pathway that regulate proliferation, survival, metastasis and angiogenesis. HER2 is an orphan receptor and does not need ligand binding for activation. HER2 is also the preferred dimerisation partner for other HER family member proteins. The humanized monoclonal antibody pertuzumab binds HER2 at a region distinct from that of trastuzumab and prevents HER2 dimerisation (A). Similarly, the humanized monoclonal antibody trastuzumab binds HER2, leading to inhibition of downstream signaling in a mechanism that is not fully understood (B). Lapatinib is a reversible small-molecule tyrosine kinase inhibitor that has dual activity against the tyrosine kinase ATP-binding pocket of HER1 and HER2 (C). The antibody-drug conjugate TDM-1 combines trastuzumab linked to the microtubulin-disruptive agent emtansine (DM1). Trastuzumab binds HER2-overexpressing cells and is internalized via endocytosis. Emtansine is released, causing inhibition of microtubule assembly and regulation of cellular processes (D).