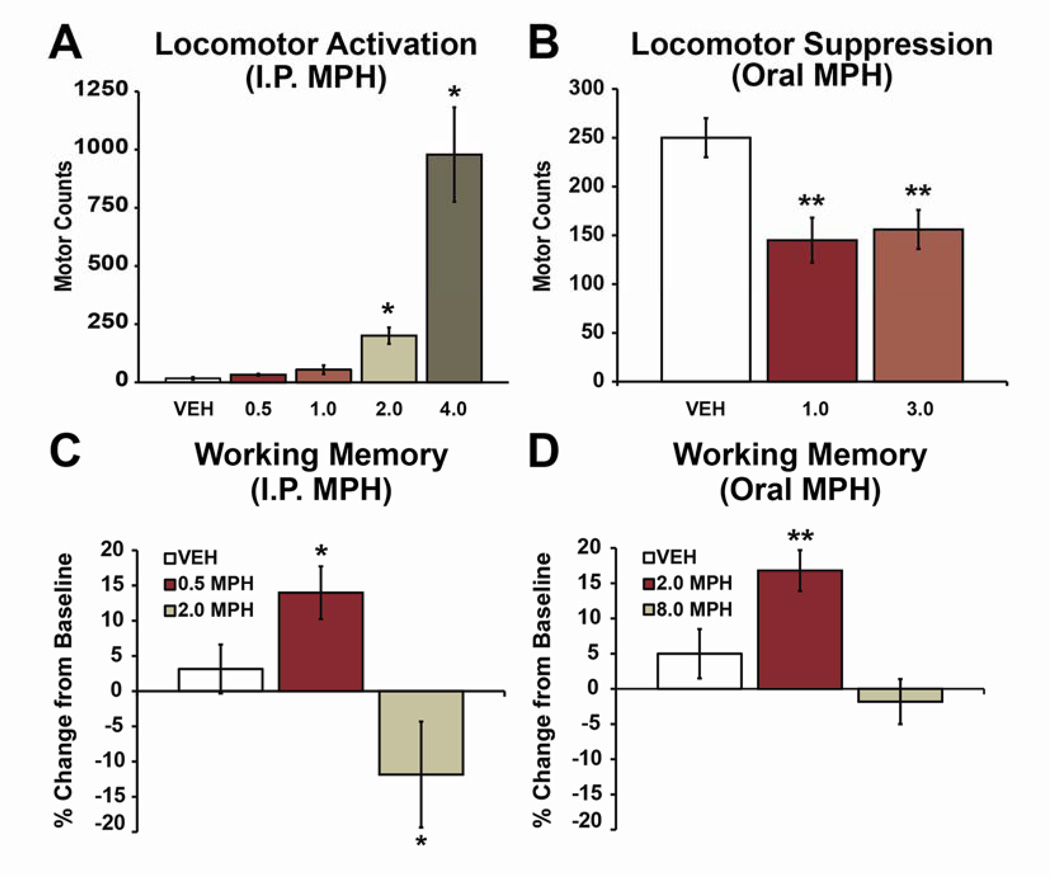
Fig 1. Behavioral-calming and cognition-enhancing actions of clinically-relevant doses of methylphenidate (MPH) in rats.
A: Lack of locomotor activation. Bars indicate motor counts (quadrant entries + rears) in the 90-minute period following subcutaneous treatment with vehicle (VEH) or varying doses of MPH (0.5, 1.0, 2.0 and 4.0 mg/kg) during the light (inactive) phase of the circadian cycle in rats (i.e. under low arousal conditions). Doses that produce clinically-relevant plasma concentrations (0.5, 1.0 mg/kg) do not elicit significant locomotor activation. Higher doses elicit dose-dependent increases in locomotor activity. The 4.0 mg/kg dose was peri-threshold for eliciting mild stereotypy. B: Motoric calming. When tested under conditions associated with elevated motor activity (during the dark/active phase) clinically relevant doses of oral MPH (1.0, 3.0 mg/kg) suppressed motor activity, similar to that seen in ADHD patients. C, D: Cognition enhancement. When administered in doses that elicit clinically relevant plasma concentrations, intraperitoneally (C) or orally (D) administered MPH improves working memory performance as measured by percent change from baseline performance in a delayed-response test of working memory (T-maze). 4-fold higher doses impair or do not improve performance. All bars indicate mean ± SEM. *P<0.05, **P <0.01 compared with vehicle-treated animals. Modified from (6,24,33).