Cone dystrophy with supernormal rod response (CDSRR) (RCD3B, OMIM #610356) was first described in 2 siblings by Gouras et al1 in 1983. In subsequent reports, it has been characterized as a rare autosomal recessive retinal disorder associated with a delayed and markedly decreased cone and rod response that exhibits an exaggerated, or superthreshold, rod electroretinogram (ERG) in response to higher stimulus levels.2 Reports of CDSRR commonly describe an early onset of dyschromatopsia, photophobia, and central scotoma with poor best-corrected visual acuity.3 Associated signs and symptoms include nyctalopia, nystagmus, and macular retinal pigment epithelium changes.3,4
Genetic studies have linked CDSRR to mutations in the potassium channel, subfamily V, member 2 gene (KCNV2), which is predominantly expressed in retinal photoreceptors and encodes a modulatory subunit of theKv8.2 voltage-gated potassium channel.4 Mutations in KCVN2 may inhibit proper assembly of heteromeric voltage-gated potassium channels with a subsequent pathologically prolonged outward potassium current in the dark, causing an abnormality in photoreceptor membrane potentials.5 The exclusive link of CDSRR to KCNV2 mutations is a notable contrast from the majority of inherited retinal disorders, which display genetic heterogeneity.
Methods
A complete ophthalmic examination by a retinal physician (S.H.T.) was performed, including fundus autofluorescence using scanning laser ophthalmoscopy (Heidelberg retinal angiograph; Heidelberg Engineering), microperimetry (MP1; Nidek Technologies), and spectral-domain optical coherence tomography (Spectralis optical coherence tomography/scanning laser ophthalmoscopy; Heidelberg Engineering). An electrophysiological assessment was performed using the Espion 5 system (Diagnosys). Full-field electroretinograms (ERGs) were recorded according to the standards of the International Society for Clinical Electrophysiology of Vision.
Blood samples were genetically screened at Casey Eye Institute Laboratory, Portland, Oregon, for KCVN2 coding region mutations that cause disease. All exons and flanking introns of KCVN2 were directly sequenced on the ABI 3100XL DNA sequencer (Applied Biosystems), and detected variants were analyzed for evolutionary conservation by the prediction programs PolyPhen-2 and SIFT.
Report of a Case
A 47-year-old man presented with decreased visual acuity and a history of hemeralopia and photophobia for several years. Medical, ocular, and family histories were unremarkable. Best-corrected visual acuity was 20/150 OD and 20/100OS. Pupils, extraocular movements, confrontational visual fields, intraocular pressures, and anterior segment examination findings were within normal limits. Color vision was diminished to 3/6 on Hardy-Rand-Rittler plates in both eyes.
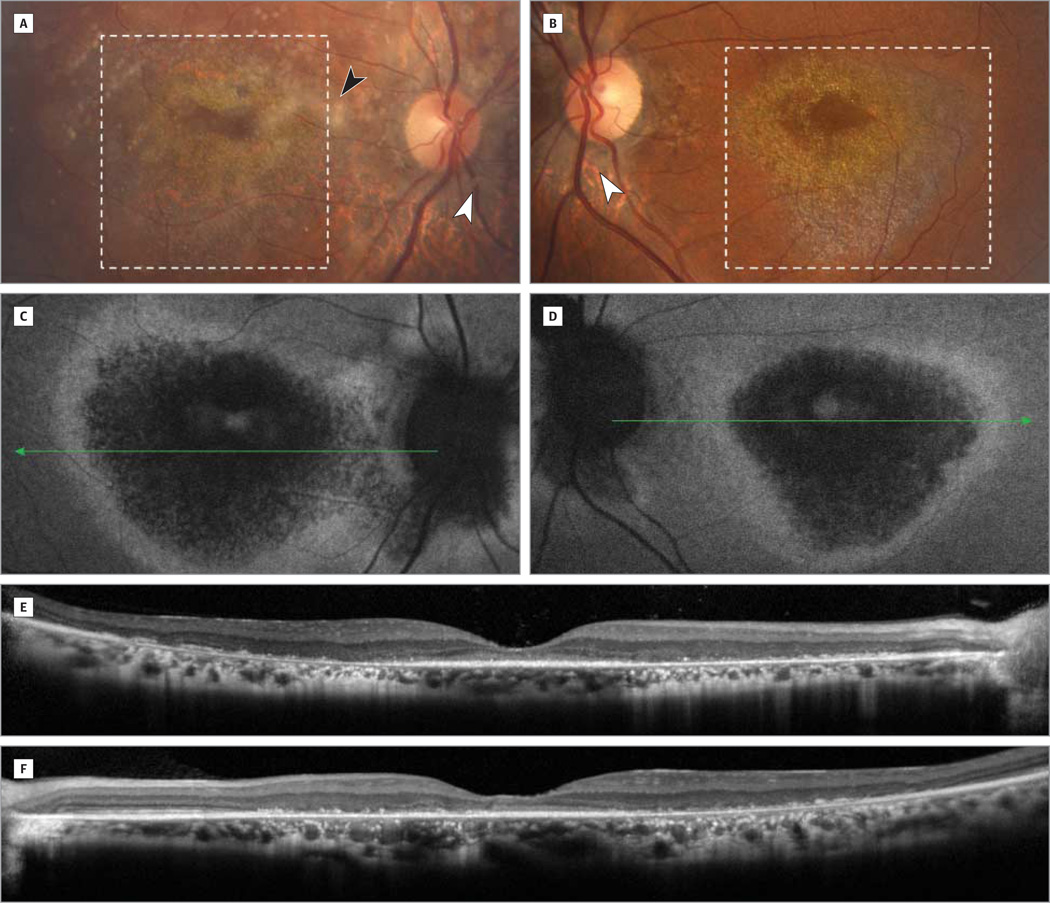
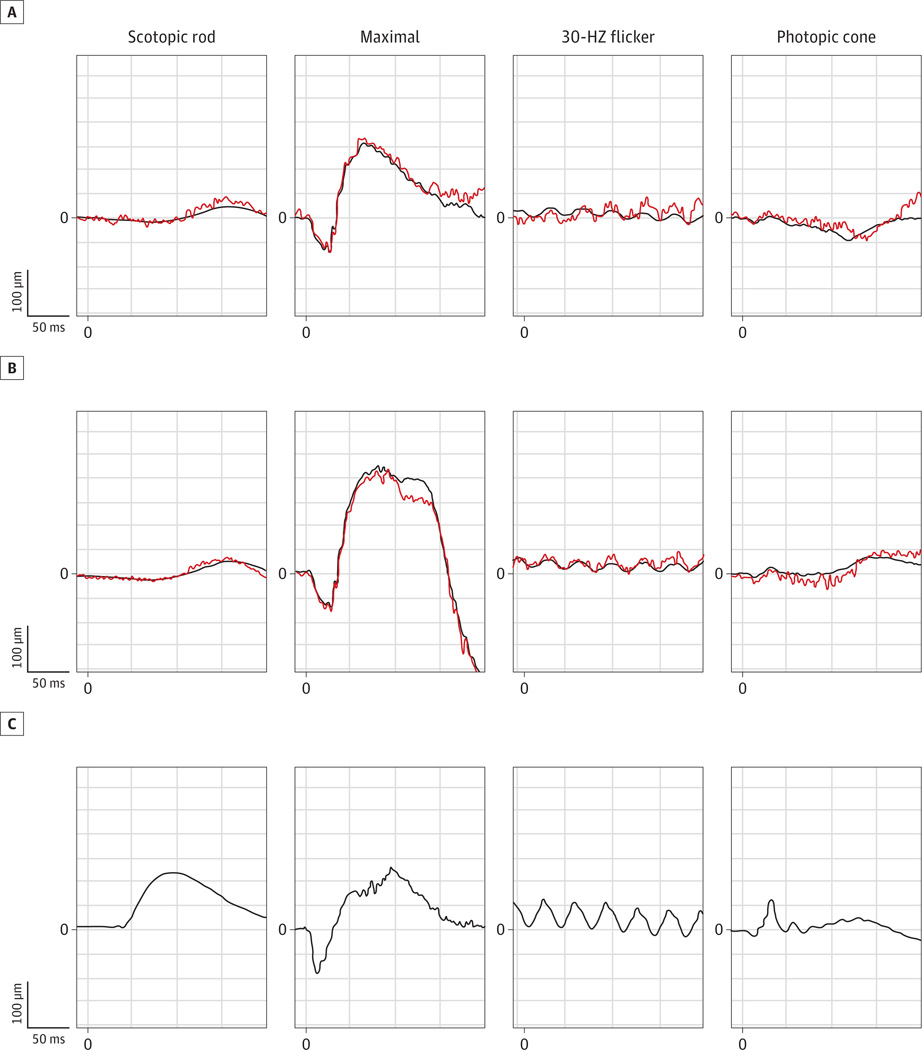
On fundus examination, there was bilateral macular atrophy with retinal pigment epithelium changes and pale optic nerves with peripapillary atrophy (Figure 1A and B). The right eye had moderately dense asteroid hyalosis (Figure 1A). Fundus autofluorescence demonstrated bilateral patchy, central hypoautofluorescence surrounded by a ring of high-density hyperautofluorescence in the macula (Figure 1C and D). Spectral-domain optical coherence tomography revealed diffuse outer retinal atrophy as evidenced by loss of inner segment–outer segment continuity and loss of outer nuclear layer–outer plexiform layer normal architecture as well as granular changes corresponding to the hyperreflective crystalline lesions seen on fundus examination (Figure 1E and F). Mean full-field ERG results and respective stereotypical tracings showed significant delays and amplitudinal loss in the photopic cone and 30-Hz flicker ERGs indicating generalized cone system dysfunction (Figure 2). Bipolar cell-corresponding b-waves were reduced compared with the photoreceptor-specific a-wave. The dim-flash, scotopic rod ERGs (0.01 candela · s/m2) showed profoundly delayed b-waves, and the bright-flash, maximum cone and rod ERGs (11 candelas · s/m2) showed a significantly increased amplitude along with a prolonged b-wave.
Figure 1. Color Photographs, Fundus Autofluorescence Images, and Optical Coherence Tomographic Images.
Color photographs of the right (A) and left (B) eyes demonstrate bilateral macular atrophy (dotted inset) with pale optic nerves with peripapillary atrophy (white arrowheads) and diffuse retinal pigment epithelial changes. There was also a significant amount of asteroid hyalosis (black arrowhead) in the right eye (A). These magnified images of the macula highlight the graduated atrophy corresponding to the bright deposits around the fovea in both eyes (A and B) exhibiting a hyperreflective crystalline appearance. Fundus autofluorescence images of the right (C) and left (D) eyes demonstrate hypoautofluorescent areas suggestive of central atrophy with a surrounding ring of hyperautofluorescence corresponding to lipofuscin accumulation in the retinal pigment epithelium likely secondary to incomplete degradation of photoreceptor outer segments. These autofluorescence findings correlate with retinal optical coherence tomographic imaging of the right (E) and left (F) eyes showing diffuse outer retinal atrophy bilaterally.
Figure 2. International Society for Clinical Electrophysiology of Vision–Standardized Full-Field Electroretinograms.
The mean and typical traces in the right (A) and left (B) eyes of the patient are shown, and electroretinograms from an age-matched control subject with results within 2 SDs of the normal mean values representative of normal traces in this age group are shown for comparison (C). In the patient, photopic cone and 30-Hz Flicker responses were reduced and significantly delayed, the scotopic rod-specific response was delayed and reduced, and the maximal response was notably higher and more prolonged compared with the normative control.
The diagnosis of CDSRR was suspected based on reports of an association between this unique ERG finding andKCNV2 mutations. Genetic testing confirmed the diagnosis and also revealed 2 novel mutations, p.W46X:c.137G>A and p.C177X: c.531T>A, in KCVN2 to possibly account for this novel disease phenotype.
Discussion
There are several novel conclusions to be drawn from this case of CDSRR. Testing with ERG can focus candidate gene screening in patients with dyschromatopsia, nyctalopia, or hemeralopia presenting with distinct bilateral macular atrophy with retinal pigment epithelial changes and diffuse outer retinal atrophy. This case highlights specific optical coherence tomographic findings of diffuse outer retinal atrophy that correspond with previously described mechanistic theories detailing preservation of the inner nuclear layer.3 Additionally, these findings confirm reports in the literature of older patients presenting with fundus autofluorescence images with a ring like area of high density encircling retinal pigment epithelial atrophy.2 It is likely that in patients who present later, milder symptoms and more subtle examination findings are consistent with slowly progressing atrophy outward from the macula, highlighted by complete degradation centrally and incomplete degradation of photoreceptor outer segments in a peripheral ring.
Ensembl analysis revealed that pC177X c531T>A is likely a nonsense truncating mutation in the N-terminal, cytoplasmic tetramerization domain of theKv8.2 protein, thus impairing its ability to form functional α-unit tetrameric potassium channels (http://www.ensembl.org/).ThepW46Xc137G>Amutation is a nonsense mutation that can also lead to a truncated protein. Both are loss-of-function alleles.6
The 2 mutations identified are novel and thus expand the current knowledge of CDSRR genotype-phenotype descriptions in the literature. This patient uniquely presented with a more dramatic macular pattern at a much later age than an average patient with CDSRR and had neither nyctalopia nor dyschromatopsia. This would suggest not only that the heterogeneity of phenotypes for CDSRR is much broader than the literature indicates but also that the patient’s 2 novel mutations are mild, having resulted in compound heterozygosity associated with a late presenting phenotype.
Acknowledgments
Funding/Support: This work was supported by the LuEsther T. Mertz Retinal Research Center, Manhattan Eye, Ear, and Throat Hospital, Macular Foundation, Inc, core grant 5P30EY019007 from the National Eye Institute, core grant 5P30CA013696 from the National Cancer Institute, and unrestricted funds from Research to Prevent Blindness. The Bernard and Shirlee Brown Glaucoma Laboratory, Department of Ophthalmology, Columbia University is supported by grant R01 EY018213 from the National Institutes of Health, the Foundation Fighting Blindness, Schneeweiss Stem Cell Fund, and the Tistou and Charlotte Kerstan Foundation. Tsang is a member of the RD-CURE Consortium, is a fellow of the Burroughs-Wellcome Program in Biomedical Sciences, and has been supported by the Bernard Becker Association of University Professors in Ophthalmology Award from Research to Prevent Blindness, the Dennis W. Jahnigen Award from the American Geriatrics Society, the Joel Hoffman Scholarship, the Barbara and Donald Jonas Family Fund, and the Professor Gertrude Rothschild Stem Cell Foundation.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Study concept and design: Dhrami-Gavazi, Lee, Mukkamala, Tabacaru, Yannuzzi, Gouras, Tsang.
Acquisition of data: Lenis, Dhrami-Gavazi, Lee, Mukkamala, Tabacaru, Yannuzzi, Tsang.
Analysis and interpretation of data: Lenis, Dhrami-Gavazi, Lee, Mukkamala, Tabacaru, Yannuzzi, Tsang.
Drafting of the manuscript: Lenis, Dhrami-Gavazi, Lee, Mukkamala, Tabacaru, Gouras, Tsang.
Critical revision of the manuscript for important intellectual content: Lenis, Lee, Mukkamala, Yannuzzi.
Obtained funding: Lenis, Mukkamala.
Administrative, technical, or material support: Dhrami-Gavazi, Lee, Tabacaru, Yannuzzi, Tsang.
Study supervision: Mukkamala, Gouras, Tsang.
Conflict of Interest Disclosures: None reported.
Contributor Information
Tamara Lee Lenis, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, Ohio.
Elona Dhrami-Gavazi, Department of Ophthalmology, Columbia University, New York, New York.
Winston Lee, Department of Ophthalmology, Columbia University, New York, New York; Department of Pathology and Cell Biology, Columbia University, New York, New York.
Sri Krishna Mukkamala, Department of Ophthalmology, Columbia University, New York, New York; Vitreous Retina Macula Consultants of New York, New York.
Mirela Raluca Tabacaru, Department of Ophthalmology, Columbia University, New York, New York.
Lawrence Yannuzzi, Vitreous Retina Macula Consultants of New York, New York.
Peter Gouras, Department of Ophthalmology, Columbia University, New York, New York.
Stephen H. Tsang, Department of Ophthalmology, Columbia University, New York, New York; Department of Pathology and Cell Biology, Columbia University, New York, New York.
References
- 1.Gouras P, Eggers HM, MacKay CJ. Cone dystrophy, nyctalopia, and supernormal rod responses: a new retinal degeneration. Arch Ophthalmol. 1983;101(5):718–724. doi: 10.1001/archopht.1983.01040010718003. [DOI] [PubMed] [Google Scholar]
- 2.Robson AG, Webster AR, Michaelides M, et al. “Cone dystrophy with supernormal rod electroretinogram”: a comprehensive genotype/phenotype study including fundus autofluorescence and extensive electrophysiology. Retina. 2010;30(1):51–62. doi: 10.1097/IAE.0b013e3181bfe24e. [DOI] [PubMed] [Google Scholar]
- 3.Michaelides M, Holder GE, Webster AR, et al. A detailed phenotypic study of “cone dystrophy with supernormal rod ERG.”. Br J Ophthalmol. 2005;89(3):332–339. doi: 10.1136/bjo.2004.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wissinger B, Dangel S, Jägle H, et al. Cone dystrophy with supernormal rod response is strictly associated with mutations in KCNV2. Invest Ophthalmol Vis Sci. 2008;49(2):751–757. doi: 10.1167/iovs.07-0471. [DOI] [PubMed] [Google Scholar]
- 5.Wissinger B, Schaich S, Baumann B, et al. Large deletions of the KCNV2 gene are common in patients with cone dystrophy with supernormal rod response. Hum Mutat. 2011;32(12):1398–1406. doi: 10.1002/humu.21580. [DOI] [PubMed] [Google Scholar]
- 6.Smith KE, Wilkie SE, Tebbs-Warner JT, et al. Functional analysis of missense mutations in Kv8.2 causing cone dystrophy with supernormal rod electroretinogram. J Biol Chem. 2012;287(52):43972–43983. doi: 10.1074/jbc.M112.388033. [DOI] [PMC free article] [PubMed] [Google Scholar]