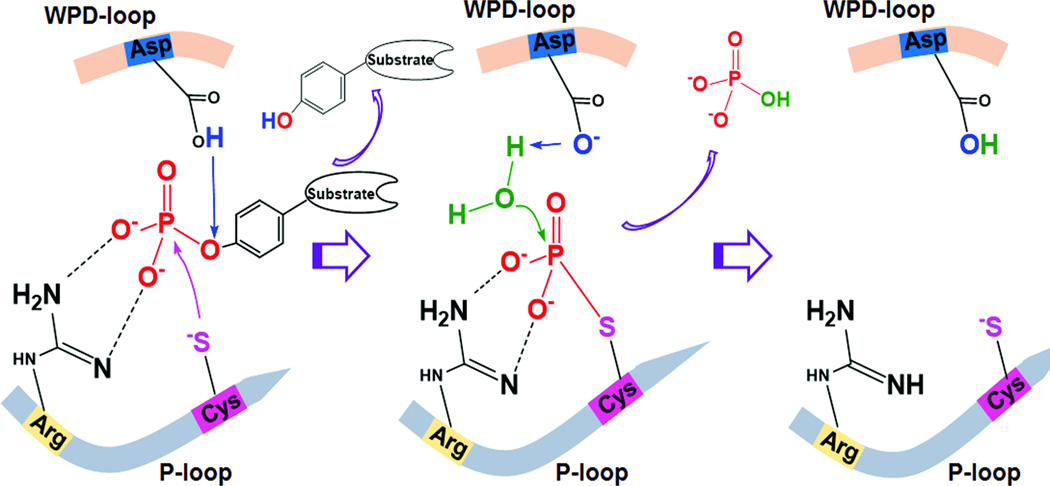
Figure 2. Catalytic mechanism of PTPs.
The catalytic cysteine in the P-loop initiates the nucleophilic attack of the phosphorous atom on pY and thus breaks the phosphorus–oxygen bond, whereas the catalytic aspartate in the WPD loop acts as a generate acid to donate a proton to the dephosphorylated tyrosine. This step generates a phosphocysteine intermediate and releases the dephosphorylated substrate. This phosphocysteine intermediate is then cleaved by the action of the catalytic aspartate, which acts as a general base to extract a proton from a water molecule and facilitates the hydrolysis of the phosphorous-sulfur bond. This reaction results in the release of free phosphate.