Abstract
Background
The ecological plaque hypothesis for the etiopathogenesis of caries implies a microbial shift towards a more aciduric dental plaque microbiota, due to a frequent carbohydrate intake. Acid tolerance has been suggested as an important property of the caries-associated bacteria and several in vitro studies with mixed cultures indicated that a low pH rather than the carbohydrate availability is responsible for microbiota shifts associated with the development of dental caries.
Objective
To examine 1) the acidogenic potential (amount lactate produced per mg plaque and minute, at pH 7.0 or pH 5.5) and the aciduric potential (acidogenic potential at pH 5.5/acidogenic potential at pH 7.0) of dental plaque and salivary sediment taken from caries-active or caries-free adults, and 2) the effect of a short-term chlorhexidine treatment on these potentials.
Design
Dental plaque and saliva sediment samples were taken from caries-free and caries-active subjects and suspended in Ringer's solution containing 1% sucrose and buffered with 0.5 M 3-[N-morpholino]propanesulfonic acid (MOPS), pH 7.0, or 3-[N-morpholino]ethanesulfonic acid (MES), pH 5.5. After incubation at 37°C for 10–20 min, the concentration of lactic acid in the suspension was determined by an enzymatic assay. The acid production of dental plaque was also determined after a period of mouth rinsing with 0.2% chlorhexidine.
Results
Both dental plaque and salivary sediment from caries-free subjects exhibited significantly lower acidogenic potentials at both pHs compared to caries-active volunteers. The opposite was observed with the aciduric potential. Chlorhexidine treatment significantly reduced all three potentials but had no effect on the relative proportion of bacteria grown on acidic agar.
Conclusions
Caries-active adults have an oral microbiota characterised by an increased catabolic velocity for sugar. The increase is more pronounced at neutral than acidic pH. Exposure to chlorhexidine, through mouthwash, temporarily decreases the acidogenicity of the microbiota.
Keywords: acidurance, acidogenicity, caries, chlorhexidine, dental plaque, saliva sediment
According to the ecological plaque hypothesis for the etiopathogenesis of dental caries, the frequent intake of fermentable carbohydrates causes a shift in the composition of the oral microbiota towards an increase of the proportion of aciduric bacteria (1, 2). Furthermore, the extended form of this hypothesis considers dynamic changes that allow expression of aciduric characteristics by the existing microbiota besides the establishment of new aciduric species in the oral ecosystem (3, 4). These microbial changes are suggested to result in an increased acid production in dental plaque, especially at low pH, which accelerates caries formation.
Certain aciduric bacteria such as lactobacilli and mutans streptococci have been correlated with caries activity and were considered as the main culprit of dental caries (5–9). However, results from studies using molecular identification techniques also indicated disease association with other bacterial communities, including some novel species (10–14), while Streptococcus mutans could not be detected in 10–20% of patients with severe caries activity (15, 16).
Higher concentrations of lactate, the main acidic end product in dental plaque exposed to excess of sugars, have been found in caries-active than caries-inactive subjects (17–20) and caries activity has been associated with a faster pH drop and a lower minimal pH in dental plaque (10, 21). Although these findings indicate differences in the catabolic activity of the oral microbiota between caries-active and inactive people, they do not necessarily prove the shift towards a more aciduric microbial community as the main ecological change as implied by the ecological plaque hypothesis.
In a recent study conducted in children (22), caries activity was found to correlate with the lactate producing velocity of dental plaque not only in an acidic environment but also in a neutral one. Moreover, the difference in catabolic velocity found at neutral pH between the caries-active and caries-free children was much higher than the corresponding one at acidic pH. Based on their findings, the authors suggested a modification of the ecological plaque hypothesis. According to this, the frequent sugar exposure primarily favours bacteria that are good sugar exploiters characterised by an increased catabolic capacity, and secondarily aciduric bacteria, the pH strategists that retain certain catabolic activity at low pH, at oral sites where acidic conditions prevail for time periods long enough to inhibit the growth of non-aciduric species.
In the present study, we aimed to examine the correlation between the caries activity and the acidogenic and aciduric potentials of dental plaque and salivary bacterial sediment from adults. Furthermore, the effect of daily chlorhexidine exposure on the acidogenic potential was studied.
Material and methods
Subjects
Thirty adults 26–64 year-old (mean age 32.8) participated in the study. All of the volunteers declared to be fit and healthy. They received no medication other than painkillers occasionally. They visited the School of Dentistry in Thessaloniki, Greece for routine examination. People with xerostomia were excluded. The subjects were randomly selected as they were visiting the clinic. After having been examined, the volunteers were asked for participation in the study and informed written consent was obtained from all of them. The study was approved by the Ethics Committee of the Dental School of the University of Thessaloniki.
Dental examination was performed by the same dentist, to determine the index Decayed Missing Filled Teeth (DMFT), using a dental mirror and an explorer under the dental chair's light. Dental radiographs were taken if necessary. Half of the subjects recruited (15 adults) were caries-free but could have some old restorations performed more than a year ago, while the remaining 15 adults had active, un-treated, caries lesions.
Sample preparation
The subjects refrained from tooth brushing for 2–3 days and were asked not to consume any food or drink except water, 3 h prior to plaque collection. Dental plaque was scraped off with a scaler from all accessible tooth surfaces and placed in a pre-weighed plastic tube. The plaque amount was determined to the nearest mg and the sample was suspended in salt solution containing (per liter) 0.6 g CaCl2, 2.1 g KCl, 5.0 g NaCl, and 0.05 g MgSO4 · 7H2O, with a glass mortar to yield a homogeneous suspension of 15 mg plaque/ml.
On the same occasion and immediately after the plaque collection, a 10-ml stimulated saliva sample was received from each volunteer, after chewing paraffin wax. The samples were centrifuged and the sediment was suspended in 1 ml salt solution as described above.
Determination of acidogenic and aciduric potentials
To an aliquot of plaque suspension or salivary sediment suspension, an equal volume of salt solution containing 1 M buffer and 2% sucrose was added. The buffer compound used in the neutral reaction mixtures was 3-[N-morpholino]propanesulfonic acid (MOPS), pH 7.0, and in the acidic mixtures 3-[N-morpholino]ethanesulfonic acid (MES), pH 5.5. The high concentration of the buffers secured a stable pH during the incubation period. The suspensions were incubated aerobically, at 37°C, for 10 min (neutral mixtures) or 20 min (acidic mixtures). At the end of the incubation, the mixtures were placed in an ice-water bath for 10 min to stop the fermentation and centrifuged (10,000×g, 5 min). The supernatants were aspirated and stored at −75°C until analysed. The whole procedure was completed within 1 h from the sample (plaque or saliva) collection.
The concentration of L-lactic acid in the supernatants was determined with an enzymatic method using a commercially available reagent kit (Sentinel Diagnostics, Sentinel CH, Milan, Italy). In brief, the concentration of lactate was measured by the increase in absorbance at 340 nm as a consequence of the conversion of NAD to NADH together with the formation of pyruvate from lactate in the presence of lactate dehydrogenase. The acidogenic potential is defined as the amount (in mg) of lactic acid per mg plaque and minute of incubation, detected at either neutral or acidic pH. The ratio of the acidogenic potential at pH 5.5 by the acidogenic potential at pH 7.0 is defined as the aciduric potential (22).
Effect of chlorhexidine
To examine the effect of chlorhexidine, the caries-active subjects were asked to rinse their mouth twice daily with 10 ml 0.2% chlorhexidine digluconate solution for 10 days. Plaque samples were taken before the rinsing period (baseline), after the rinsing period (after having refrained from toothbrushing and the chlorhexidine mouthwash for 3 days) and 1 month later (45 days from baseline). The samples were processed as described above for determining their acidogenic and aciduric potentials.
Microbiological analysis
A 50-µl aliquot of the plaque samples taken on the three occasions (before and after the chlorhexidine rinsing period) was serially diluted with salt solution. From appropriate dilutions, a small amount (0.1 ml/ plate) was inoculated on the culture media Brucella agar (BA) and Mitis Salivarius agar (MS). Two variants of BA were used. The neutral BA (BAN) had pH 7.0, while the acidic BA (BAA) had its pH adjusted to 5.5 by the addition of lactate before sterilisation.
All plates were incubated at 37°C, in air with 5% CO2 for 2 days. Thereafter, the number of colonies was counted to determine the total facultative anaerobic microbes (grown on BAN), the aciduric facultative anaerobic microbes (grown on BAA) and the streptococci (grown on MS).
Statistical analysis
The data were analysed with the ANOVA test to examine the correlation of the dental plaque/saliva potentials between the caries-active and caries-free groups. Pearson's correlation two-tailed test was used to depict the correlation among dental plaque potentials and caries lesions. The differences between the potentials and the logarithms of the bacterial counts as well were assessed with Student's t-test. The software PASW Statistics 18 (SPSS Inc., Hong Kong, China), formerly SPSS Statistics, was used for the analysis of the data.
Results
Acidogenicity and acidurance
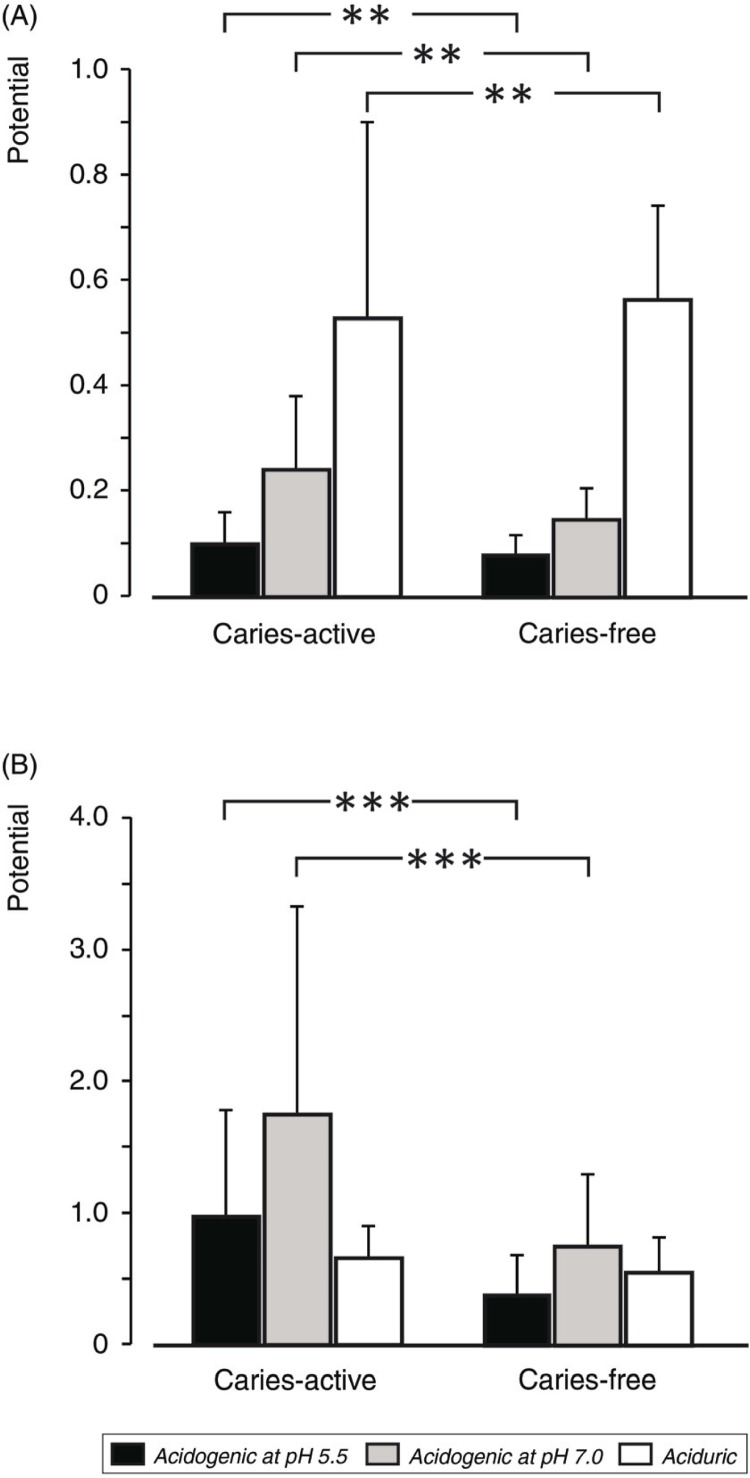
In the subjects with no caries experience, the mean acidogenic potential of dental plaque at pH 7.0 was 0.15 mg lactate/mg plaque×min (range: 0.06–0.25). At pH 5.5, the acidogenic potential ranged from 0.04 to 0.15 with a mean of 0.08 (Fig. 1A). In the caries-active adults (mean DMFT: 4), the corresponding figures were 0.24 (range: 0.06–0.53) at pH 7.0 and 0.10 (range: 0.03–0.22) at pH 5.5. The acidogenic potentials were significantly (p<0.01) higher for the caries-active than the caries-free group (Fig. 1A).
Fig. 1.
Acidogenic (at pH 7.0 and pH 5.5) and aciduric potentials of supragingival plaque (A) and saliva sediment (B) from caries-free and caries-active adults. Significant differences are indicated. **p<0.01, **p<0.001.
A slight difference was also found for the aciduric potential between the groups (Fig. 1A). However, the caries-free subjects exhibited a slightly higher aciduricity than the caries-active group, this difference being dependent on the much lower acidogenic potential at pH 7.0 recorded for the caries-free subjects.
The potentials measured for saliva sediments were significantly (p<0.001) higher than those of dental plaque (Fig. 1B). The highly significant (p<0.001) differences between the caries-active and caries-free subjects were also larger than the respective data for plaque samples. For the former group, the acidogenic potentials at pH 7.0 and 5.5 were 1.74 (range: 0.22–4.66) and 0.97 mg lactate/ml sediment suspension×min (range: 0.19–2.69), respectively. For the caries-free subjects, the corresponding figures were 0.75 (range: 0.10–2.19) and 0.37 (range: 0.003–1.12). The aciduric potential for the saliva sediment was similar in both groups (Fig. 1B).
Correlation analysis between the caries activity (number of decayed teeth) and the various potentials revealed a weak correlation (r=0.5, p<0.05) with only the acidogenic potential at pH 5.5 of the baseline plaque samples from the caries-active subjects.
Effect of chlorhexidine
Rinsing with the chlorhexidine mouthwash for 10 days significantly (p<0.05) reduced the acidogenic and aciduric potential of the dental plaque (Fig. 2). However, the potentials returned to the baseline levels after the lapse of 1 month without chlorhexidine rinsing.
Fig. 2.
Acidogenic (at pH 7.0 and pH 5.5) and aciduric potentials of supragingival plaque from caries-active adults before (baseline), 2 days after [after chlorhexidine (CHX)], and 1 month after daily mouth rinses with 0.2% chlorhexidine solution for 1 week. Significant differences are indicated. *p<0.05.
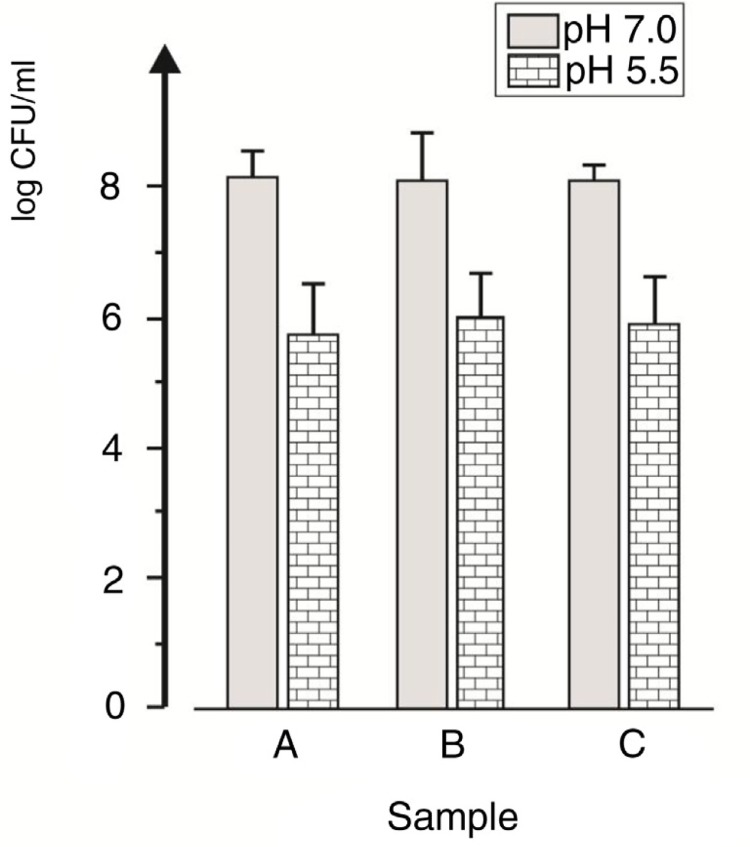
The lowered acidogenicity and aciduricity were not reflected in the total numbers of facultative anaerobic bacteria that grew on the neutral (BAN) and the acidic (BAA) agar plates (Fig. 3). The colony counts on BAA were 10–100 times lower than those on BAN, this difference being significant (p<0.001). However, no other difference was observed in the microbial counts of dental plaque sampled before and after the chlorhexidine rinsing period.
Fig. 3.
Total counts (log CFU/ml) of facultatively anaerobic bacteria grown on BAN (pH 7.0) and BAA (pH 5.5) media in plaque samples taken before (A), 2 days after (B), and 1 month after (C) the rinsing period with 0.2% chlorhexidine.
Streptococci counted on MS agar plates comprised about half of the sample microbiota (the total facultative anaerobic bacteria grown on BAN) on all three occasions. No significant difference was found in their proportions in relation to the chlorhexidine rinsing.
Discussion
In line with previous findings (18, 19, 21, 23), the oral microbiota of patients with active carious lesions exhibits a higher capacity for lactate production from sugar compared with caries-free adults. This is evident at both neutral and acidic pH; however, the greatest difference in acidogenic capacity between the two groups appears at neutral pH. The same trend, albeit with more intense differences, was earlier observed with dental plaque samples from children (22). A similar tendency is also seen in the correlation between the caries activity expressed as decayed teeth and the lactate producing velocity although in adults the significant correlation was only found with the acidogenic potential at pH 5.5.
The differences in acidogenicity and acidurance between the caries-free and caries-active subjects were more pronounced with the salivary sediments than the plaque. Regarding acidogenicity, a possible explanation for the differences could be a higher number of bacteria in the sediments than in plaque. Nevertheless, the higher bacterial number cannot explain the differences in acidurance. It is plausible to assume that a different composition in bacterial species between the sediments and plaque may mainly account for the increased potentials of the salivary sediments.
The terms aciduric and acid tolerant have been used in the past to characterise bacteria that either survive exposure to acids or have catabolic activity and can grow at low pH (24, 25). Depending on the method used, the bacterial acid resistance may be expressed as growth rate or production rate of end products or survival time in an acidic environment. Presently, we defined bacterial acidurance as a relative acidogenic activity in order to distinguish the increased acidogenic activity at low pH from an overall increased acidogenic activity. In this respect, the findings of this study indicate that the ecological shift in the oral microbiota of caries-active subjects is towards a totally increased acidogenic capacity, this being more strongly manifested at neutral than at acidic pH. Thus, the frequent exposure of the mouth to sugars, as anticipated for caries-active people, probably favours microbial species that can more efficiently utilise sugar at any pH and not only in an acidic environment.
Caries-associated bacteria, such as mutans streptococci and lactobacilli are suggested to proportionally increase in dental plaque of patients, mainly due to their acidurance (26, 27). Besides being acid tolerant, mutans streptococci are efficient sugar exploiters, exhibiting much higher catabolic velocity at neutral than at acidic pH, compared with other non-caries-associated oral streptococci (28). In vitro studies with mixed continuous cultures revealed that lactobacilli could predominate S. mutans at pH 5 or below (29), while other observations indicate that the growth rates of some lactobacilli and oral streptococci may be similar at around pH 5 (30). However, the in vivo conditions are far too different than those in vitro. For instance, the type of carbohydrate is suggested not to affect the proportions of individual species at neutral pH (26) but the culture medium may do, as in the case of sorbitol-fermenting lactobacilli that cannot utilise sorbitol when grown in a saliva-based medium (31).
Another point of concern is the acidic environment. In most studies mentioned above, pH below 4.5 are used for exposure periods of 1 h or more to induce acid tolerance or to study the effect of acid stress, while in cultures, the exposure periods to low pH equals those of incubation to achieve growth of more than 10 generations. It might be argued that these conditions are not representative of those mostly prevailing in vivo. The pH drop in dental plaque after a sugar challenge is certainly affected by the environment. The hard surface supporting the plaque may affect the pH drop, as in the case of natural tooth tissue that releases hydroxyl groups upon its dissolution and buffers the acidity around pH 5.0–5.5 (32). On the contrary, insoluble ceramic or metallic artificial tooth surfaces or those of telemetry pH electrodes will have no similar effect and may allow a stronger pH drop. The drop and the duration of low pH depend on local salivary film velocity and specific anatomical features that affect acid and sugar clearance (33, 34). Further, the duration of acidic (pH<6) conditions is often shorter than the bacterial generation time in the oral cavity (35, 36), while adaptation to acidic environments may occur in many oral bacteria within a 1-h exposure to low pH (25). On the basis of the above, it appears plausible to suggest that acidurance alone may not be a sufficient characteristic that confers an ecological advantage to certain bacteria and allow their predominance in the caries-active mouth, although in oral sites with long lasting low pH, the growth of non-aciduric bacteria may selectively be suppressed.
Rinsing with chlorhexidine for 1 week reduced the total acidogenic capacity of dental plaque. The decreased acidogenicity was observed in samples harvested after a 3-day period without tooth brushing and chlorhexidine exposure; however, the number of bacteria able to grow on acidic agar remained unchanged throughout the experimental period. Without any other intervention, the acidogenic capacity of dental plaque almost reached the baseline values within 1 month. Chlorhexidine is known to decrease the numbers of cariogenic bacteria, especially mutans streptococci, for periods up to 3 months, and its use in caries prevention has been suggested although the clinical evidence from long-term studies is insufficient (37–39). A mechanism for the preventive action of chlorhexidine may rely on the decrease of the acidogenic capacity of dental plaque bacteria, as presently shown. It is also possible that this effect of chlorhexidine is relatively stronger against bacteria with high acidogenic capacity.
Based on the present findings and those previously reported and mentioned above, we suggest the following modification of the ecological plaque hypothesis. The frequent intake of fermentable carbohydrates results in a totally longer exposure of the oral microbiota to carbohydrate concentrations that primarily favour bacteria with an increased catabolic capacity or fermentation velocity. At sites where the acidic environment created by the bacterial activity can last for sufficient time period, aciduric bacteria may be favoured by the suppressed growth of non-aciduric species.
Acknowledgements
We are grateful to Assoc. Professor Christos J. Emmanouilides for helping with the statistical analysis.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors. The study was funded by the School of Dentistry of Aristotle University of Thessaloniki, Greece.
References
- 1.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–71. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 2.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–94. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 2008;42:409–18. doi: 10.1159/000159604. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2010;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 5.Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans . Microbiol Rev. 1980;44:331–84. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loesche JW. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stecksén-Blicks C. Lactobacilli and Streptococcus mutans in saliva, diet and caries increment in 8- and 13-year-old children. Scand J Dent Res. 1987;95:18–26. doi: 10.1111/j.1600-0722.1987.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 8.Beighton D, Hellyer PH, Lynch EJ, Heath MR. Salivary levels of mutans streptococci, lactobacilli, yeasts, and root caries prevalence in non-institutionalized elderly dental patients. Community Dent Oral Epidemiol. 1991;19:302–7. doi: 10.1111/j.1600-0528.1991.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65:1028–37. [PubMed] [Google Scholar]
- 10.van Houte J, Sansone C, Joshipura K, Kent R. In vitro acidogenic potential and mutans streptococci of human smooth-surface plaque associated with initial caries lesions and sound enamel. J Dent Res. 1991;70:1497–502. doi: 10.1177/00220345910700120501. [DOI] [PubMed] [Google Scholar]
- 11.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–81. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 12.van Houte J, Lopman J, Kent R. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res. 1996;75:1008–14. doi: 10.1177/00220345960750040201. [DOI] [PubMed] [Google Scholar]
- 13.Sansone C, van Houte J, Joshipura K, Kent R, Margolis HC. The association of mutans streptococci and non-mutans streptococci capable of acidogenesis at a low pH with dental caries on enamel and root surfaces. J Dent Res. 1993;72:508–16. doi: 10.1177/00220345930720020701. [DOI] [PubMed] [Google Scholar]
- 14.Beighton D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol. 2005;33:248–55. doi: 10.1111/j.1600-0528.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 15.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–9. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–17. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geddes DAM. Acids produced by human dental plaque metabolism in situ . Caries Res. 1974;9:98–109. doi: 10.1159/000260149. [DOI] [PubMed] [Google Scholar]
- 18.Minah GE, Loesche WJ. Sucrose metabolism in resting-cell suspensions of caries-associated and non-caries-associated dental plaque. Infect Immun. 1977;17:43–54. doi: 10.1128/iai.17.1.43-54.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao XJ, Fan Y, Kent RL, Jr, van Houte J, Margolis HC. Association of caries activity with the composition of dental plaque fluid. J Dent Res. 2001;80:1834–39. doi: 10.1177/00220345010800091201. [DOI] [PubMed] [Google Scholar]
- 20.Margolis HC, Moreno EC. Composition and cariogenic potential of dental plaque fluid. Crit Rev Oral Biol Med. 1994;5:1–25. doi: 10.1177/10454411940050010101. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi K, Hamada Y, Nishimaki H, Sakurai S, Kamiyama K. The acidogenic potential of plaque from sound enamel, white spot lesions, and cavities in children. Pediatr Dent. 1987;9:212–15. [PubMed] [Google Scholar]
- 22.Andreadis G, Kalfas S. Correlation of dental plaque acidogenicity and acidurance with caries activity – perspectives of the ecological plaque hypothesis. J Adv Med Res. 2014;1:49–55. [Google Scholar]
- 23.Englander HR, Carter WJ, Fosdick LS. The formation of lactic acid in dental plaques. J Dent Res. 1956;35:792–9. doi: 10.1177/00220345560350052001. [DOI] [PubMed] [Google Scholar]
- 24.Svensäter G, Larsson UB, Greif EC, Cvitkovitch DG, Hamilton IR. Acid tolerance response and survival by oral bacteria. Oral Microbiol Immunol. 1997;12:266–73. doi: 10.1111/j.1399-302x.1997.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Yamada T. Acid-induced acid tolerance and acidogenicity of non-mutans streptococci. Oral Microbiol Immunol. 1999;14:43–8. doi: 10.1034/j.1399-302x.1999.140105.x. [DOI] [PubMed] [Google Scholar]
- 26.Marsh PD. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proc Finn Dent Soc. 1991;87:515–25. [PubMed] [Google Scholar]
- 27.Bowden GH, Hamilton IR. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and “S. mitior” growing in continuous culture. Can J Microbiol. 1987;33:824–7. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- 28.de Soet JJ, Nyvad B, Kilian M. Strain-related acid production by oral streptococci. Caries Res. 2000;34:486–90. doi: 10.1159/000016628. [DOI] [PubMed] [Google Scholar]
- 29.Bowden GH, Hamilton IR. Competition between Streptococcus mutans and Lactobacillus casei in mixed continuous culture. Oral Microbiol Immunol. 1989;4:57–64. doi: 10.1111/j.1399-302x.1989.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 30.Horiuchi M, Washio J, Mayanagi H, Takahashi N. Transient acid-impairment of growth ability of oral Streptococcus, Actinomyces, and Lactobacillus: a possible ecological determinant in dental plaque. Oral Microbiol Immunol. 2009;24:319–24. doi: 10.1111/j.1399-302X.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- 31.Kalfas S, Edwardsson S. Effect of culture medium on acid production from sorbitol by oral bacteria. Acta Odontol Scand. 1990;48:217–22. doi: 10.3109/00016359009005877. [DOI] [PubMed] [Google Scholar]
- 32.Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69:722–4. [PubMed] [Google Scholar]
- 33.Macpherson LM, Dawes C. Effects of salivary film velocity on pH changes in an artificial plaque containing Streptococcus oralis, after exposure to sucrose. J Dent Res. 1991;70:1230–4. doi: 10.1177/00220345910700090101. [DOI] [PubMed] [Google Scholar]
- 34.Dawes C. Why does supragingival calculus form preferentially on the lingual surface of the 6 lower anterior teeth? J Can Dent Assoc. 2006;72:923–6. [PubMed] [Google Scholar]
- 35.Beighton D, Smith K, Hayday H. The growth of bacteria and the production of exoglycosidic enzymes in the dental plaque of macaque monkeys. Arch Oral Biol. 1986;31:829–35. doi: 10.1016/0003-9969(86)90137-8. [DOI] [PubMed] [Google Scholar]
- 36.Sissons CH, Wong L, Cutress TW. Patterns and rates of growth of microcosm dental plaque biofilms. Oral Microbiol Immunol. 1995;10:160–7. doi: 10.1111/j.1399-302x.1995.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 37.Emilson CG. Potential efficacy of chlorhexidine against mutans streptococci and human dental caries. J Dent Res. 1994;73:682–91. doi: 10.1177/00220345940730031401. [DOI] [PubMed] [Google Scholar]
- 38.Bowden GH. Mutans streptococci caries and chlorhexidine. J Can Dent Assoc. 1996;62:703–7. [PubMed] [Google Scholar]
- 39.Autio-Gold J. The role of chlorhexidine in caries prevention. Oper Dent. 2008;33:710–6. doi: 10.2341/08-3. [DOI] [PubMed] [Google Scholar]