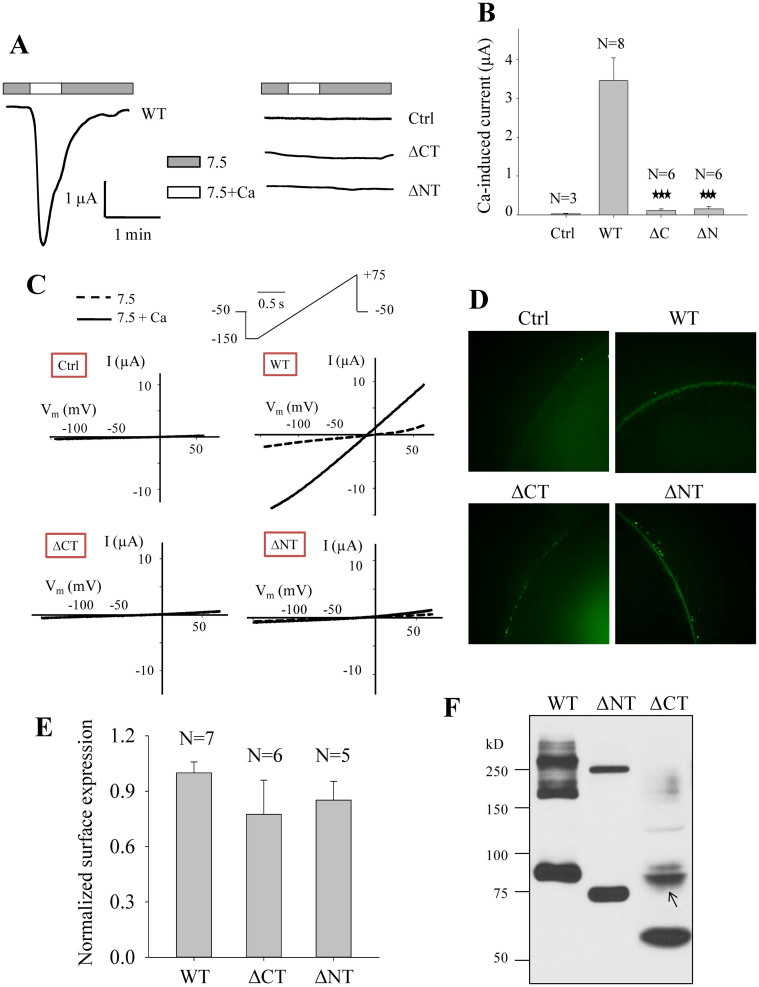
Figure 2. Roles of the human PKD2L1 N- and C-termini in its channel activity and oligomerization.
(A) Representative whole-cell current tracings obtained from Xenopus oocytes expressing PKD2L1 WT, mutant ΔCT (deletion of E566-S805) or ΔNT (deletion of M1-Y96) using the two-microelectrode voltage clamp technique. Oocytes were voltage clamped at −50 mV. Data from a water-injected oocyte served as a negative control (Ctrl). Currents were measured using standard extracellular solution (pH 7.5) (7.5) or standard extracellular solution containing 5 mM CaCl2 (7.5+Ca). (B) Averaged currents obtained from oocytes expressing PKD2L1 WT, ΔCT, ΔNT or water (Ctrl). Currents were averaged from different numbers of oocytes, as indicated. ‘***' indicates p ≤ 0.001 when compared with the WT data. (C) Representative current–voltage relationship curves obtained using a voltage ramp protocol, as indicated, before (7.5) and after (7.5+Ca) addition of 5 mM CaCl2. (D) Representative immunofluorescence data showing the plasma membrane expression of PKD2L1 WT, ΔCT and ΔNT in oocytes. (E) Averaged and normalized surface expression of PKD2L1 WT, ΔCT and ΔNT in oocytes. Surface expressions were averaged from indicated numbers of oocytes and normalized to that of PKD2L1 WT. (F) WB detection of Flag-tagged human PKD2L1 WT, ΔNT and ΔCT over-expressed in HeLa cells under the non-reducing condition. A band (indicated by an arrow) that is unlikely a dimer based on its size remained unaccounted for was detected with the CT deletion.