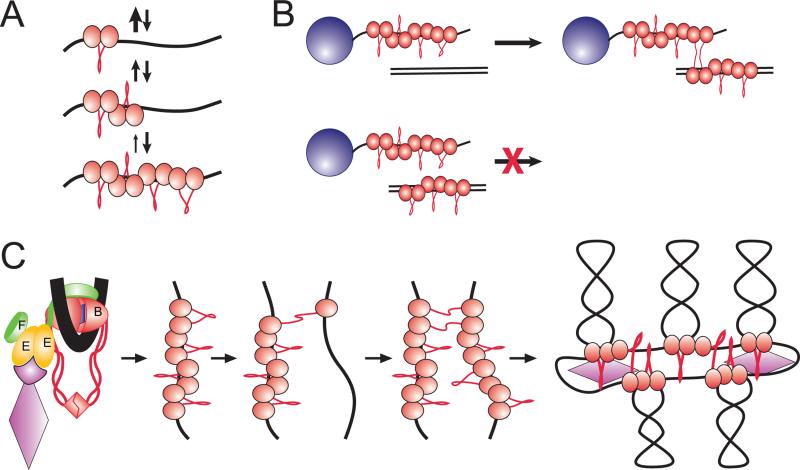
Figure 5. DNA scaffolding activity of MukBEF.
(A) Nucleation-propagation mechanism of DNA binding. A single MukB forms only a short-lived complex with DNA. The binding, however, is highly cooperative, and each subsequent bound protein stabilizes the cluster through nearest neighbor interactions.
(B) Bridging occurs between DNA bound MukB clusters and naked DNA.
(C) Proposed mechanism of chromosome organization. The protein is postulated to be recruited to its initial binding site with the help of the regulatory subunits. The protein binds the target site via the clamping mechanism described in (A). Once the clamp is formed, the MukB cluster attempts bridging with randomly colliding DNA segments. Finding a sufficiently long stretch of naked DNA allows stable bridging and further spreading of the scaffold.