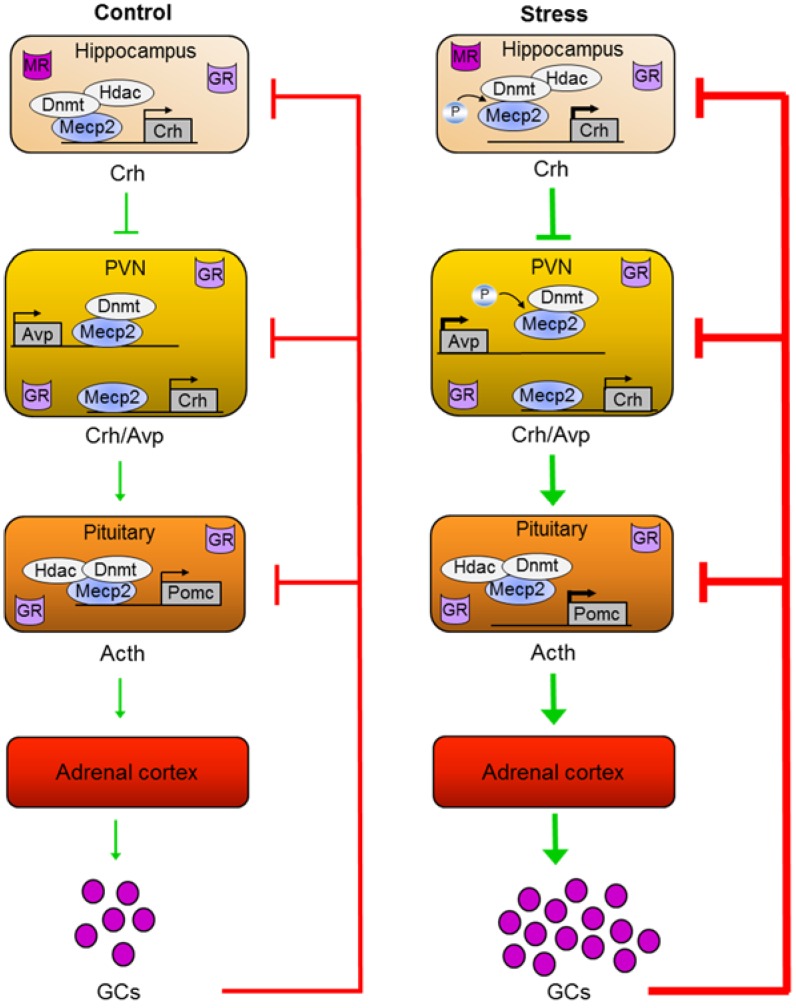
Figure 2.
Roles of Mecp2 in epigenetic programming of the HPA axis by ELS. The two neuropeptides corticotrophin-releasing hormone (Crh) and arginine-vasopressin (Avp) are released in response to stress from the nucleus paraventricularis of the hypothalamus (PVN) and jointly stimulate in the anterior pituitary the production of pro-opiomelanocorticotrophin precursor mRNA (Pomc) and the secretion of its posttranslational product adrenocorticotrophin (Acth). Subsequently, Acth enhances the secretion of glucocorticoids (GCs) from the adrenals. These stress hormones bind to nuclear glucocorticoid and mineralocorticoid receptors (GR and MR), which are expressed at different levels of the HPA axis and serve to set back the stress response. In hippocampal CA3 Mecp2 binds under resting conditions to the Crh promoter and represses gene activity through recruitment of Dnmts and Hdacs. Upon ELS exposure, Mecp2 becomes phosphorylated at S421, dissociates, and relieves Crh repression. Mecp2 also binds to Crh in the PVN but stays unaffected by ELS. In contrast, Mecp2 occupancy at the downstream Avp enhancer responds to ELS by S421-phosphorylation and derepression of Avp. Due to its association with Dnmts, Mecp2 occupancy maintains enhancer methylation, while ELS-induced dissociation facilitates hypomethylation and gives rise to a lasting memory trace. Likewise, Mecp2 binds to the proximal Pomc promoter region in the anterior pituitary and interacts hereby with Dnmts and Hdacs to repress Pomc expression. In contradistinction to the PVN, ELS-induced Mecp2 dissociation is unrelated to S421-phoshorylation indicating tissue- and cell-type specificity of this modification. Loss of Mecp2 binding facilitates, however, Pomc hypomethylation and increased gene expression.