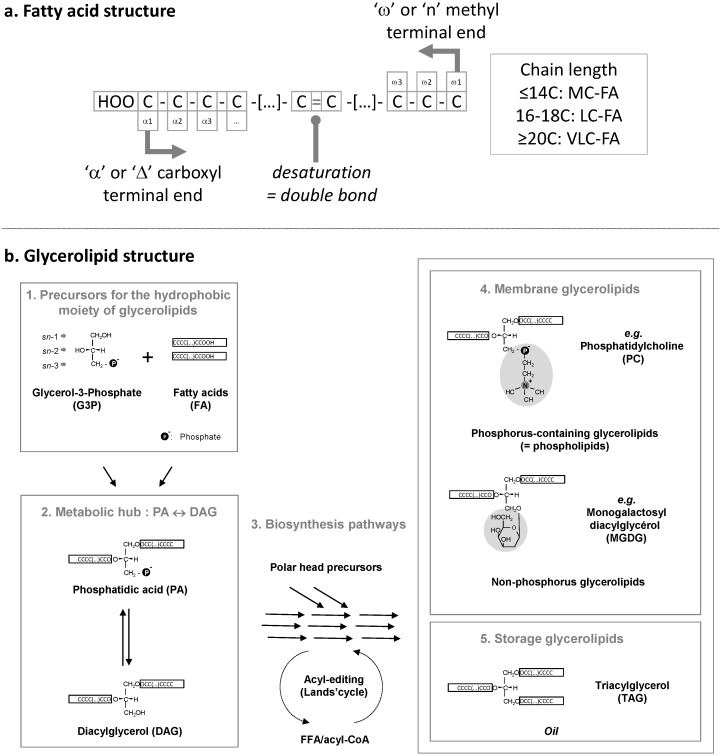
Figure 1.
(a) Schematic structure of a fatty acid. Carbons are numbered either starting from the carboxyl terminal end (“α” or “Δ” nomenclature) or from the methyl terminal end (“ω” or “n” nomenclature). The chain length can vary. MC, medium chain; LC, long chain; VLC, very long chain; FA, fatty acid; (b) Incorporation of fatty acids in glycerolipids. Initial precursors (1), i.e., glycerol-3-phosphate (G3P) and fatty acids (FA) are used to produce phosphatidic acid (PA) and its dephosphorylated form diacylglycerol (DAG), which are at the origin of all glycerolipids. Glycerolipid biosynthesis pathways (3) comprise multiple reactions leading to the production of membrane polar glycerolipids (4), or storage triacylglycerol (5). The sn-1, sn-2, and sn-3 numbering of the glycerol backbone is shown. This scheme gives an example of a phospholipid, phosphatidylcholine (PC), synthesized in the endoplasmic reticulum, and an example of a non-phosphorus glycolipid, monogalactosyldiacylglycerol (MGDG), synthesized in the chloroplast. It is important to note that exchanges of FAs can occur in some lipids, like PC, via a process known as acyl-editing. A PC molecule can be hydrolyzed into Lyso-PC, releasing a FA, and re-acylated using another acyl-CoA. The complete de-acylation/re-acylation process is called the Lands cycle and does not imply any net production of glycerolipid. Neo-synthesized FAs can be massively incorporated into glycerolipids at this step.