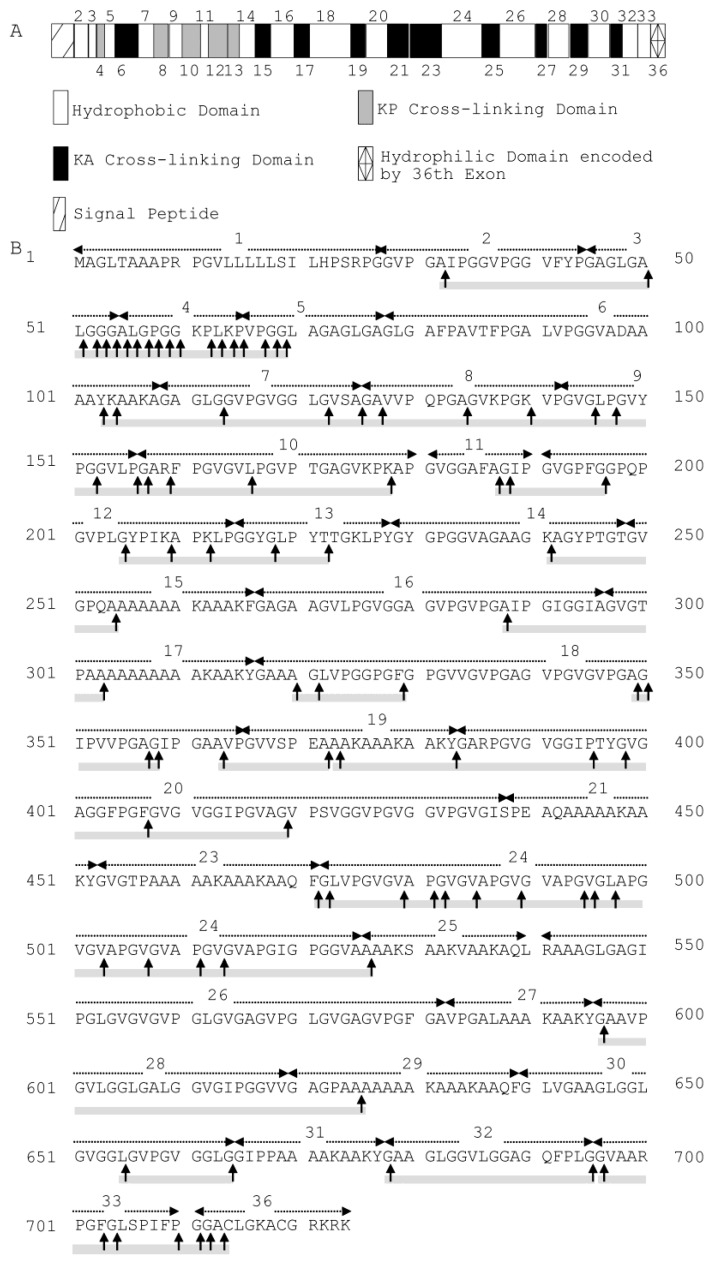
Figure 6.
(A) Domain map of human tropoelastin containing all possible exons. The hydrophilic cross-linking domains are further divided into KP cross-linking domains, with lysine pairs separated by one or more proline residues, and KA cross-linking domains, with lysine pairs separated by alanine residues. Domain 36 is assigned differently because of its unique structural features [2]. (B) Cleavage sites of myroilysin in recombinant human tropoelastin. Each dashed arrow above the sequence indicates a domain encoded by an individual exon. The cleavage sites are marked by vertical arrows. The cleavage sites were determined based on the sequences of peptides from recombinant human tropoelastin released by myroilysin, as shown in Supplementary Table S2. The sequences covered by the determined peptides are underlined with grey solid lines.