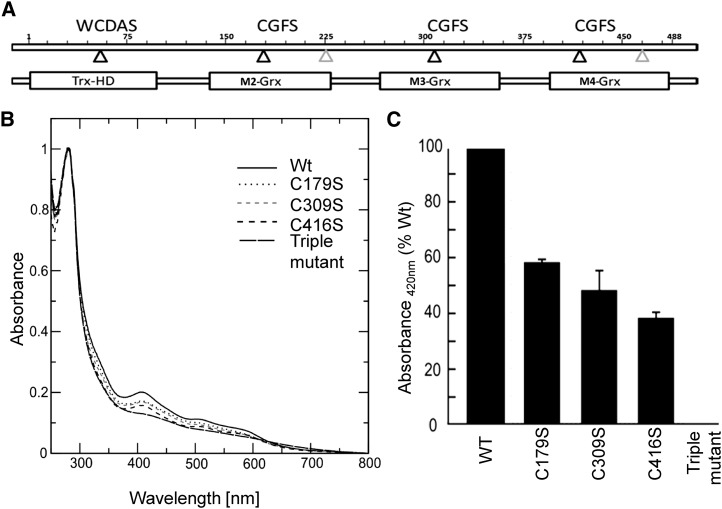
Figure 5.
Incorporation of iron-sulfur clusters into recombinant wild-type and mutated AtGRXS17. A, Domain structure of GRXS17. Positions of active-site cysteines are indicated by black triangles, and positions of other Cys are indicated by gray triangles. M2-GRX, M3-GRX, and M4-GRX are three monothiol-GRX domains. TRX-HD, TRX-like homology domain; WCDAS. B, Absorption spectra of GRXS17 and Cys mutants. UV-visible absorption spectra were recorded immediately after in vitro reconstitution in anaerobic conditions. The active-site cysteines of each GRX domain were individually or together substituted by Ser (M2:C179S, M3:C309S, M4:C416S, and C179/309/416S). C, Relative absorption at 420 nm of GRXS17 mutants. WT, Wild type.