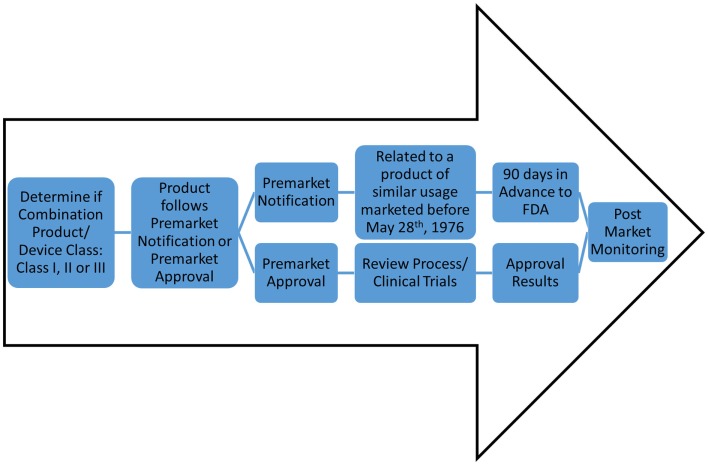
Figure 2.
Potential FDA regulatory routes of decellularized matrices. For classification as a device, there are classes of device safety standards ranging from Class I (e.g., toothbrush) to Class III (e.g., pacemaker). However, an engineered organ will likely be classified as a combination product. Determination of product as Pre-market Notification or Pre-market Approval will allow for one of two routes: (1) 90 days notification to the FDA for market (for devices for similar use as products pre-dating May 28th, 1976), or (2) clinical trials and approval process; respectively. Both routes, once marketed and commercially available, will undergo stringent post-market monitoring.