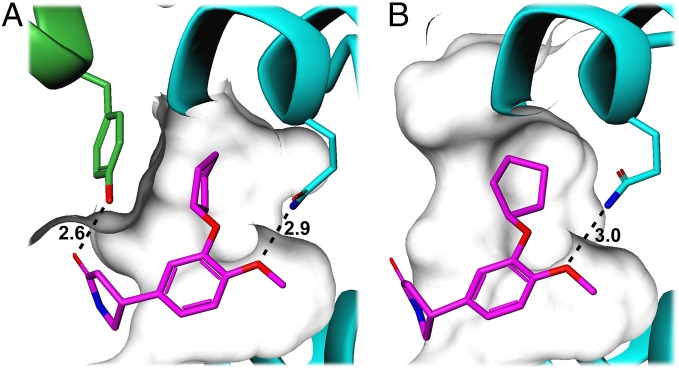
Fig. 6.
High- and low-affinity binding modes of rolipram. The structures of rolipram (purple sticks) bound to (A) PDE4Bcryst and to (B) PDE4B catalytic domain (PDB ID code 1XMY) were superimposed by minimizing the rmsd of the Cα atoms of the catalytic domains, and are shown here in the same orientation in two separate figures for clarity. Hydrogen bonding interactions are shown as dashed lines, with distances labeled in angstroms. Both structures show a hydrogen-bond with the conserved glutamine at the base of the substrate-binding pocket. In the high-affinity binding mode (A), Tyr274 from the regulatory helix forms one wall of the hydrophobic pocket occupied by the cyclopentyl group, and its hydroxyl oxygen forms a hydrogen bond with the amide carbonyl oxygen in rolipram. Both these interactions are absent in the low-affinity binding mode shown in B.