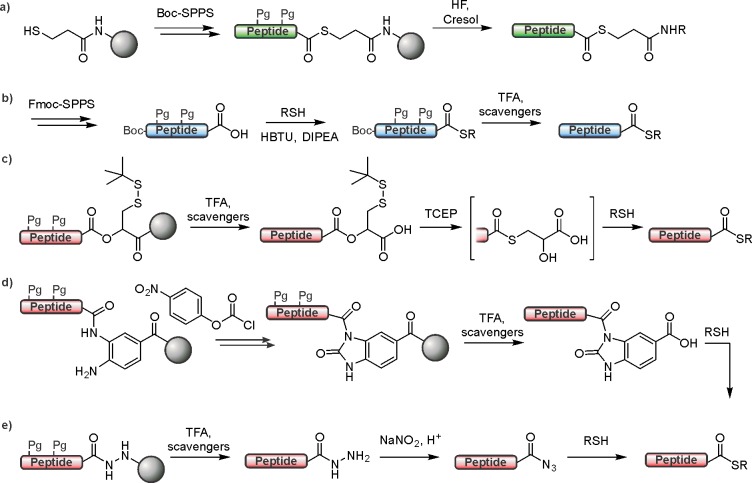
Figure 39.
Comparison of peptide α-thioester synthesis by Boc- (a) and Fmoc-SPPS (b–e). (a) Synthesis of peptide α-thioesters on a mercaptopropionic acid linker by Boc-SPPS. (b) Direct conversion of a protected peptide acid into an α-thioester. (c) Latent thioester synthesis on a 2-hydroxy-3-mercaptopropionic acid linker. (d) α-Thioester synthesis through an acylthiourea intermediate. (e) Acyl-hydrazide method for α-thioester synthesis. Pg = protecting group.