Abstract
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm (MPN) characterized by excessive production of red blood cells. Patients with PV are at a risk of thrombosis, bleeding, and transformation to myelofibrosis or acute myeloid leukemia. Therapy for PV is based on the use of phlebotomy, aspirin, and in high-risk patients, cytoreductive agents such as hydroxyurea. Anecdotal evidence suggests that imatinib mesylate, a selective tyrosine kinase inhibitor of ABL1, ARG, PDGFR, and KIT kinases has activity in PV. We conducted an open-label phase II clinical trial of imatinib at the standard dose of 400 mg daily in 24 patients with PV. The median duration of imatinib therapy was 5.1 months (range 0.2–86.4). Overall, 4 (17%) patients responded: one had a complete and three partial hematological response. The median time to response was 17.5 months (range 6–28), and the median duration of response was 17 months (range 9–68). No significant changes in JAK2V617F mutation burden were noted during imatinib therapy when compared with pre-treatment values (P = 0.46). Therapy with imatinib was generally well tolerated. Our data indicate that imatinib has minimal clinical activity in PV.
Keywords: Imatinib mesylate, Polycythemia vera, JAK2 mutation
1 Introduction
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm (MPN) that arises from clonal transformation and expansion of a hematopoietic stem cell [1]. Erythrocytosis is the most outstanding feature of PV, but it is also frequently associated with increased levels of white blood cells and platelets. Current therapeutic practice is aimed at reducing the increased risk of thrombosis in these patients, and is based on the use of phlebotomy and aspirin. Hydroxyurea is a chemotherapeutic agent reserved for patients with high-risk (for thrombosis) disease. A hallmark of PV is the characteristic in vitro growth of burst forming unit erythroid (BFU-E) colonies in the absence of added erythropoietin (Epo), also known as endogenous erythroid colonies (EEC) [2]. Growth of EEC in the absence of Epo is unique to PV, and never observed in healthy individuals or other forms of erythrocytosis. Mutations in protein tyrosine kinases have a significant role in oncogenic transformation; hence, they have become an attractive therapeutic target. This is best illustrated by the efficacy in treating chronic myeloid leukemia (CML) with imatinib mesylate [3], a potent inhibitor of the fusion protein tyrosine kinase BCRABL1, and an inhibitor of platelet-derived growth factor receptors (PDGFRs), KIT, and ARG (ABL1-related gene) protein tyrosine kinases [4]. Growth inhibition of EEC, as well as enhanced sensitivity of ex vivo expanded erythroid progenitors obtained from patients with PV to imatinib has been reported in 2003 [5, 6]. Several reports followed indicating that imatinib may have therapeutic benefit for selected patients with PV [7–10]. PDGFRs and KIT have been postulated as a possible biological targets of imatinib therapy, responsible for clinical observations. Between 2001 and 2006, we conducted a phase II clinical trial of imatinib for patients with PV at our institution.
The identification in 2005 of a somatic activating mutation in the JH2 pseudokinase auto-inhibitory domain of JAK2 (JAK2V617F) tyrosine kinase provided critical insight into the pathogenetic lesions involved in the development of the PV phenotype [11–14]. This mutation causes constitutive activation of JAK2 resulting in cytokine independent (or hypersensitive) cell growth, and constitutive activation of signal transduction pathways important for cell survival and proliferation [12]. Transplantation of lethally irradiated mice with murine marrow cells carrying mutant JAK2V617F resulted in a PV-like phenotype characterized by erythrocytosis and evolution to myelofibrosis [15]. In contrast to other MPNs, more than 90% of patients with PV harbor the JAK2V617F mutation, in both heterozygous and homozygous forms [16, 17]. In 2006, it was reported that imatinib did not have an effect on JAK2 tyrosine kinase, at that point our trial closed. Here, we present clinical and molecular results obtained in a cohort of 24 patients with PV treated with imatinib.
2 Patients and methods
Patients with PV, defined according to 2001 WHO criteria, were included in this prospective single institution trial. Patients had to have symptoms and signs of the disease requiring therapy. Patients were allowed to enter the study on a stable dose of hydroxyurea, provided its dosage had been stable for at least 4 weeks before study entry. Other inclusion criteria were Eastern Cooperative Oncology Group performance status <3, AST and ALT ≤2.5 the upper limit of normal (ULN), bilirubin ≤1.5 times the ULN, serum creatinine ≤2.5 times the ULN, age <18 years, and life expectancy <12 weeks. The protocol was approved by the Institutional Review Board of M.D. Anderson Cancer Center. All patients signed a written informed consent. Starting dose of imatinib was 400 mg orally daily. If no response was observed after 3 months, or there was evidence of disease worsening, imatinib could be increased to 800 mg orally daily. Patients had complete blood count and metabolic panel checked every week in the first month of treatment and every 2–4 weeks subsequently. Bone marrow aspirate and biopsy were performed on study every 3–6 months. JAK2 mutational burden was recently determined in stored bone marrow aspirate samples (when available) obtained from patients on study, using quantitative allele-specific suppressive PCR as previously described [18].
Complete hematological response (CHR) was defined as (1) sustained hematocrit <45% in men and <42% in women over 3 months period, without phlebotomy requirements, (2) normal white blood cell and platelet count, and (3) absence of palpable splenomegaly. Partial hematological response (PHR) required first criteria (of the 3 listed above) to be satisfied, with variable response to the second and third criteria. Concomitant use of any therapy (including hydroxyurea) for PV other than imatinib precluded response evaluation. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0.
3 Results
3.1 Study group
Between June 2001 and March 2006, 24 patients were enrolled (Table 1). Median number of prior therapies was 2 (range 1–3), including phlebotomy (n = 22), hydroxyurea (n = 13), anagrelide (n = 10), and radioactive phosphorus (n = 1). Five patients were treated with phlebotomy alone. Four patients entered the study on a stable dose of hydroxyurea, but still requiring phlebotomy. Bone marrow examination was compatible with a diagnosis of PV in all patients. Twenty-one patients had diploid cytogenetics and three had abnormalities: del(20q), t(2;4), and inv(9), respectively. All patients were negative by PCR for BCR-ABL1 rearrangement. Twenty patients were tested for the presence of JAK2V617F mutation and all were positive.
Table 1.
Baseline patient characteristics (n = 24)
| Characteristic | Median (range) |
|---|---|
| Age (years) | 57 (28-84) |
| Males (number) | 17 |
| Palpable spleen (number) | 11 |
| Henatocrit (%) | 46.0 (37.0-52.3) |
| WBC count (×109/L) | 12.9 (5.4-29.2) |
| Platelet count (×109/L) | 539 (201-937) |
| Cytogenetics other than diploid (number) | 3 |
| Prior disease duration (years) | 5 (0.3-35) |
| Prior therapies (number) | 2 (1-3) |
3.2 Response to imatinib therapy
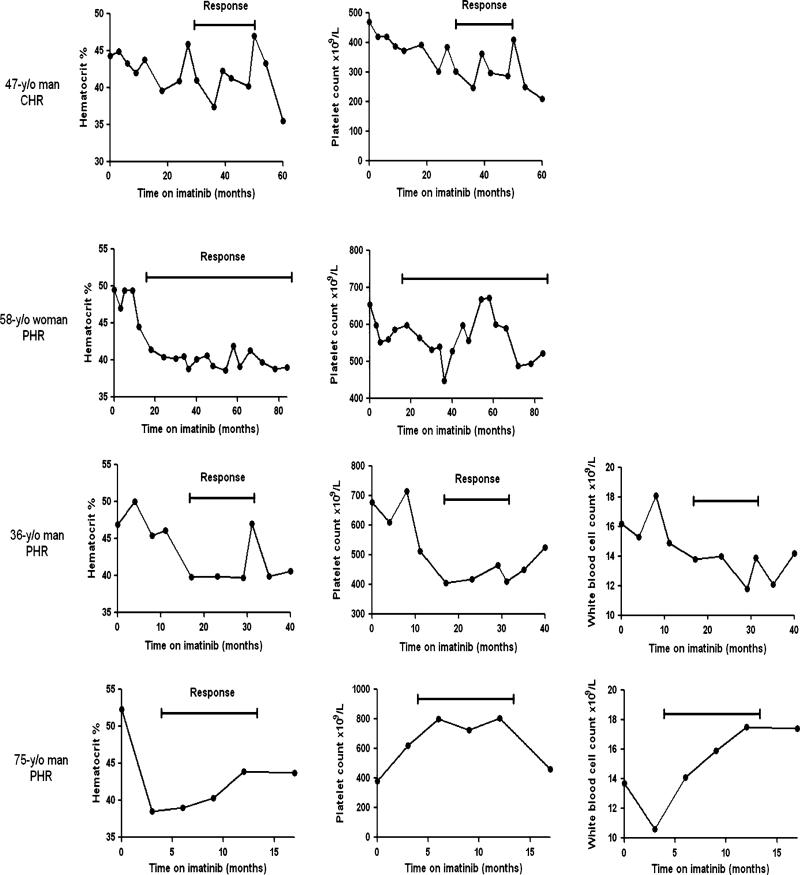
Median duration of imatinib therapy was 5.1 months (range 0.2–86.4). Four patients (17%) responded (1 CHR and 3 PHR), with median time to response of 17.5 months (range 6–28), and with median duration of response of 17 months (range 9–68). There was no statistical difference in clinical characteristics of responders and non-responders. Responding patients’ blood cell counts during the therapy are presented in Fig. 1. The patient who achieved CHR was a 47-year-old man who, on presentation to our institution, was treated with phlebotomies every 2–4 weeks, and had pruritus and splenomegaly palpable 3 cm below left costal margin on physical examination. His WBC count was 7.7 × 109/L and platelet count 470 × 109/L. His JAK2V617 mutational burden was 20%. After 12 months of imatinib 400 mg daily, he experienced grade-3 liver toxicity requiring temporary interruption of therapy. He then resumed imatinib at 300 mg daily and achieved CHR after 30 months of therapy. His symptoms resolved, spleen became non-palpable, and platelets normalized. After 20 months in CHR, however, he had to resume phlebotomy.
Fig. 1.
Clinically relevant selected blood cell counts in responding patients with polycythemia vera while on imatinib therapy
Of the three patients who had PHR, one was a 58-year-old woman who, on presentation, was treated with phlebotomies (every 2 months) and anagrelide, and complained of frequent headache. Her WBC count was normal and platelet count 654 × 109/L. Her JAK2V617 mutational burden was 23%. On imatinib 400 mg daily she achieved PHR after 18 months, with hematocrit of <42%, but with persistently elevated platelets. The dose of imatinib had to be reduced to 300 mg daily after 53 months due to nocturnal cramps and diarrhea. She is still in PHR after 68 months on imatinib therapy. The second patient was a 36-year-old man treated with phlebotomies every 3 weeks, and with enlarged spleen palpable 2 cm below the left costal margin. His WBC count was 16.2 × 109/L and platelet count 677 × 109/L. His JAK2V617 mutational burden was 82%. Imatinib dose was increased after 4 months of therapy from 400 to 800 mg daily, and then decreased after 8 months to 600 mg daily because of diarrhea. He achieved PHR on imatinib 600 mg daily after 17 months of therapy with hematocrit <45%, normal platelets, and non-palpable spleen, but his WBC remained elevated. After 14 months in PHR, he lost response. The third patient was a 75-year-old man treated with monthly phlebotomies. He had received hydroxyurea previously and had to discontinue it due to side effects. His WBC count was 13.7 × 109/L and platelet count was normal. His JAK2V617 mutational burden was 45%. He achieved PHR after 4 months of treatment with hematocrit <45%, but with increased WBC and platelets. After 9 months on imatinib, he had to stop therapy due to clotting and bleeding problems; soon afterwards he resumed phlebotomy. Three additional patients had a reduction in phlebotomy requirement by >50% and continued treatment with imatinib for 22, 33 and 36 months, respectively.
3.3 Dynamics of JAK2V617F mutation burden
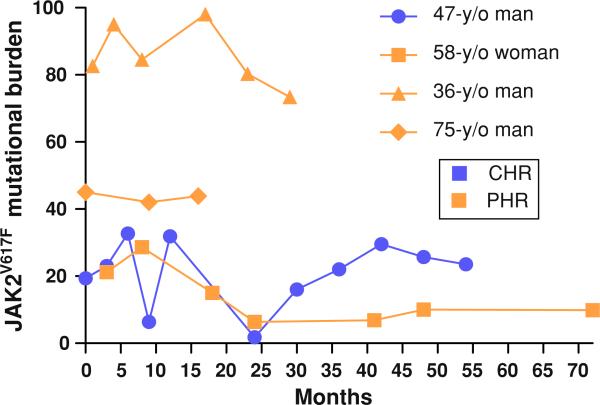
JAK2V617F mutation burden (ratio between JAK2V617F and wild-type JAK2 mRNA) was determined at the beginning, during, and the end of the treatment with imatinib in 15 patients in whom sequential stored samples were available. These patients were on imatinib therapy for a median of 9 months. The median number of available sequential samples per patient was 3. No significant changes in JAK2V617F mutation burden were noted, with a median value of 64.8% (19.4–88.2)% at the start of imatinib therapy and a median of 58.7% (9.9–89.2)% at the end of therapy (P = 0.46). Furthermore, there was no significant sustained change in the mutation burden in samples obtained from responding patients (Fig. 2).
Fig. 2.
Dynamics of JAK2V617F mutation burden in patients with polycythemia vera responding to treatment with imatinib
3.4 Toxicity profile
Imatinib did not cause significant hematological toxicity. Grade 3 non-hematological toxicity was observed in 8 (33%) patients (Table 2). No patient experienced grade 4 toxicities, but therapy had to be stopped in six patients due to toxicity. Eleven (45.8%) patients had no dose change. Ten (41.6%) patients had their imatinib dose increased to 800 mg daily. In two of the latter patients, imatinib dose was reduced back to enrollment dose (i.e. 400 mg daily). Three patients had their imatinib dose reduced to 300 mg daily, of whom one subsequently resumed dosing at 400 mg daily.
Table 2.
Toxicity profile of imatinib in polycythemia vera patients
| Adverse events | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) |
|---|---|---|---|
| Nausea | 14 (56%) | 0 | 0 |
| Diarrhea | 7 (28%) | 1 (4%) | 2 (8%) |
| Muscle cramps | 5 (20%) | 4 (16%) | 1 (4%) |
| Bone pain | 4 (16%) | 2 (8%) | 1 (4%) |
| Periorbital edema | 2 (8%) | 0 | 0 |
| Fatigue | 7 (28%) | 2 (8%) | 0 |
| Fluid retention/edema | 0 | 0 | 1 (4%) |
| Headache | 1 (4%) | 0 | 0 |
| Optic nerve edema | 0 | 0 | 1 (4%) |
| Gastroesophageal reflux | 1 (4%) | 0 | 1 (4%) |
| Liver toxicity | 0 | 0 | 1 (4%) |
| Rash | 3 (12%) | 2 (8%) | 1 (4%) |
4 Discussion
In addition to ABL1, imatinib also targets a selected array of protein tyrosine kinases, including PDGFR, ARG, and KIT [3, 19] thus, broadening its potential usefulness as a treatment for other malignancies. Importantly, therapy with imatinib has negligible effects on normal hematopoiesis [5]. The rationale for the use of imatinib in patients with PV is severalfold. First, a hallmark of the pathogenesis of PV is the formation of EECs in the absence of exogenous Epo stimulation [1]. It has been shown that imatinib inhibited the formation of autonomous EEC in ex vivo experimental systems [5]. This effect was initially ascribed to the inhibition of an unidentified kinase playing a central role in the pathogenesis of PV. Later on, imatinib was shown to specifically inhibit in a time- and dose-dependent manner the growth of reporter FDCP cells expressing the JAK2V617F [6] expressed almost universally by patients with PV [11–14, 20]. This inhibitory effect was mediated by imatinib-induced inactivation of JAK2, STAT5 and KIT proteins [6], underscoring the interplay at the molecular level of the JAK2/STAT5 and KIT pathways [21]. Furthermore, KIT-activating missense mutations (mostly mapping to the carboxy-terminal tyrosine kinase domain) have been reported in 7 (35%) out of 20 patients with PV [22] and in a murine model of PV, imatinib was more effective than dasatinib (a potent inhibitor of both ABL1 and SRC kinases) in controlling reticulocytosis and polycythemia [23]. In contrast, no effect of imatinib was observed in mice harboring wild-type JAK2 [23]. Several reports on the activity of imatinib in patients with PV have been published with contradictory results. Although some studies suggested that imatinib was effective for the management of erythrocytosis and splenomegaly in PV [7, 8, 23–25], others suggested that the activity of this agent in this setting is clinically modest and yields negligible molecular responses [26].
In the present study, we demonstrate that therapy with imatinib has limited activity on the hematopoiesis of patients with PV. We treated a total of 24 patients with JAK2V617F-positive PV with standard doses of imatinib (i.e. 400 mg daily). Virtually all patients had received several lines of therapy that included phlebotomy and some form of cytoreductive therapy. Imatinib was administered for a median of 5.1 months. Four patients (17%) responded, which included one patient who achieved CHR and three who had PHR. However, all but one patient with PHR have lost their response. The dynamics of JAK2V617F mutation burden (ratio between JAK2V617F and wild-type JAK2 mRNA), in accord with a prior report [26], remained remarkable impervious to imatinib therapy, with minimal decrements in JAK2 mutant allele burden over a median course of 9 months of therapy. Significant changes in JAK2V617F mutation burden were not observed even when only responders were evaluated. Notably, the toxicity profile of imatinib in this patient population was superimposable to that described in patients with CML in chronic phase [26], with the exception of headache and elevation of transaminases, whose incidence in our study cohort was significantly lower than that reported in CML studies [27].
At variance with our results, a recently published phase II trial has reported that imatinib at 400–600 mg daily yielded a CHR in 15 (75%) out of 20 patients with PV after 12 weeks of treatment [25]. Ten (50%) of the 20 patients enrolled remained in CHR after a median follow-up of 28 months [25]. These disparate and conflicting results are likely a direct consequence of differences in the response criteria employed in both studies. The former study evaluated response according to the Polycythemia Vera Study Group (PVSG), which defines CHR as normalization of the hematocrit and a platelet count ≤600 × 109/L in the absence of phlebotomy or anagrelide use. A PHR was defined as a hematocrit decreased from the baseline, but not meeting the PVSG criteria for CHR and requiring phlebotomy or anagrelide. In contrast, we used a much more strict set of response criteria that required normalization not only of the hematocrit but also of the platelet and white blood cell counts in the absence of phlebotomy or cytoreductive therapy, and complete resolution of splenomegaly to define CHR, while PHR required at the very least, the sustained normalization (not just a decrease from the pretreatment baseline) of hematocrit also in the absence of additional PV-directed therapy.
In summary, imatinib therapy has minimal clinical activity in PV and does not decrease the JAK2V617F mutant allele burden. A selected group of patients, however, may attain some degree of clinical benefit although this is usually transient.
Contributor Information
Roberto H. Nussenzveig, Department of Leukemia, M.D. Anderson Cancer Center, Box 428, 1515 Holcombe Boulevard, Houston, TX 77030, USA
Jorge Cortes, Department of Leukemia, M.D. Anderson Cancer Center, Box 428, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Matjaz Sever, Department of Leukemia, M.D. Anderson Cancer Center, Box 428, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Alfonso Quintás-Cardama, Department of Leukemia, M.D. Anderson Cancer Center, Box 428, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Pat Ault, Department of Leukemia, M.D. Anderson Cancer Center, Box 428, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Taghi Manshouri, Department of Leukemia, M.D. Anderson Cancer Center, Box 428, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Carlos Bueso-Ramos, Department of Hematopathology, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
Josef T. Prchal, Division of Hematology, School of Medicine, University of Utah, Salt Lake City, UT, USA
Hagop Kantarjian, Department of Leukemia, M.D. Anderson Cancer Center, Box 428, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Srdan Verstovsek, Department of Leukemia, M.D. Anderson Cancer Center, Box 428, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
References
- 1.Prchal JF, Prchal JT. Molecular basis for polycythemia. Curr Opin Hematol. 1999;6(2):100–9. doi: 10.1097/00062752-199903000-00008. doi:10.1097/00062752-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Prchal JF, Axelrad AA. Letter: bone-marrow responses in polycythemia vera. N Engl J Med. 1974;290(24):1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038–42. doi: 10.1056/NEJM200104053441402. doi:10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 4.Cortes J, Kantarjian H. Beyond chronic myelogenous leukemia: potential role for imatinib in Philadelphia-negative myeloproliferative disorders. Cancer. 2004;100(10):2064–78. doi: 10.1002/cncr.20211. doi:10.1002/cncr.20211. [DOI] [PubMed] [Google Scholar]
- 5.Oehler L, et al. Imatinib mesylate inhibits autonomous erythropoiesis in patients with polycythemia vera in vitro. Blood. 2003;102(6):2240–2. doi: 10.1182/blood-2003-03-0676. doi:10.1182/blood-2003-03-0676. [DOI] [PubMed] [Google Scholar]
- 6.Gaikwad A, et al. Imatinib effect on growth and signal transduction in polycythemia vera. Exp Hematol. 2007;35(6):931–8. doi: 10.1016/j.exphem.2007.03.012. doi:10.1016/j.exphem.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Jones CM, Dickinson TM. Polycythemia vera responds to imatinib mesylate. Am J Med Sci. 2003;325(3):149–52. doi: 10.1097/00000441-200303000-00007. doi: 10.1097/00000441-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Silver RT. Imatinib mesylate (Gleevec(TM)) reduces phlebotomy requirements in polycythemia vera. Leukemia. 2003;17(6):1186–7. doi: 10.1038/sj.leu.2402938. doi:10.1038/sj.leu.2402938. [DOI] [PubMed] [Google Scholar]
- 9.Silver RT. Treatment of polycythemia vera with recombinant interferon alpha (rIFNalpha) or imatinib mesylate. Curr Hematol Rep. 2005;4(3):235–7. [PubMed] [Google Scholar]
- 10.Spivak JL, Silver RT. Imatinib mesylate in polycythemia vera. Blood. 2004;103(8):3241. doi: 10.1182/blood-2003-12-4248. doi:10.1182/blood-2003-12-4248. author reply 3241–3242. [DOI] [PubMed] [Google Scholar]
- 11.Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 12.James C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. doi: 10.1038/nature03546. doi:10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 13.Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. doi: 10.1056/NEJMoa051113. doi:10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 14.Levine RL, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97. doi: 10.1016/j.ccr.2005.03.023. doi:10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Lacout C, et al. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108(5):1652–60. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 16.Jones AV, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106(6):2162–8. doi: 10.1182/blood-2005-03-1320. doi:10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 17.Verstovsek S, et al. JAK2V617F mutational frequency in polycythemia vera: 100%, >90%, less? Leukemia. 2006;20(11):2067. doi: 10.1038/sj.leu.2404379. doi:10.1038/sj.leu.2404379. [DOI] [PubMed] [Google Scholar]
- 18.Nussenzweig RH, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35(1):32–8. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Druker BJ, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–6. doi: 10.1038/nm0596-561. doi:10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 20.Zhao R, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280(24):22788–92. doi: 10.1074/jbc.C500138200. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiler SR, et al. JAK2 is associated with the c-kit proto-onco-gene product and is phosphorylated in response to stem cell factor. Blood. 1996;87(9):3688–93. [PubMed] [Google Scholar]
- 22.Fontalba A, et al. Identification of c-Kit gene mutations in patients with polycythemia vera. Leuk Res. 2006;30(10):1325–6. doi: 10.1016/j.leukres.2005.12.020. doi:10.1016/j.leukres.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Zaleskas VM, et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS One. 2006;1:e18. doi: 10.1371/journal.pone.0000018. doi:10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silver R, Fruchtman S, Feldman E, et al. Imatinib mesylate (Gleevec) is effective in the treatment of polycythemia vera: a multi-institutional clinical trial. Blood. 2004;104(11):189a. [Google Scholar]
- 25.Jones CM, Dickinson TM, Salvado A. Phase II open label trial of imatinib in polycythemia rubra vera. Int J Hematol. 2008;88(5):489–94. doi: 10.1007/s12185-008-0193-1. doi:10.1007/s12185-008-0193-1. [DOI] [PubMed] [Google Scholar]
- 26.Jones AV, et al. Minimal molecular response in polycythemia vera patients treated with imatinib or interferon alpha. Blood. 2006;107(8):3339–41. doi: 10.1182/blood-2005-09-3917. doi:10.1182/blood-2005-09-3917. [DOI] [PubMed] [Google Scholar]
- 27.Quintas-Cardama AC, J, Kantarjian H. Practical management of toxicities associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Clin Lymphoma Myeloma. 2008;8(Suppl. 3):S82–8. doi: 10.3816/CLM.2008.s.003. doi:10.3816/CLM.2008.s.003. [DOI] [PubMed] [Google Scholar]