Highlight
Isolation and characterization of a novel Arabidopsis mutant is reported. This has a cup-shaped leaf surface, as opposed to the flat leaves of wild-type plants.
Key words: Arabidopsis thaliana, cell proliferation, gibberellic acid (GA), leaf shape, surface curvature, TARANI.
Abstract
The leaf surface usually stays flat, maintained by coordinated growth. Growth perturbation can introduce overall surface curvature, which can be negative, giving a saddle-shaped leaf, or positive, giving a cup-like leaf. Little is known about the molecular mechanisms that underlie leaf flatness, primarily because only a few mutants with altered surface curvature have been isolated and studied. Characterization of mutants of the CINCINNATA-like TCP genes in Antirrhinum and Arabidopsis have revealed that their products help maintain flatness by balancing the pattern of cell proliferation and surface expansion between the margin and the central zone during leaf morphogenesis. On the other hand, deletion of two homologous PEAPOD genes causes cup-shaped leaves in Arabidopsis due to excess division of dispersed meristemoid cells. Here, we report the isolation and characterization of an Arabidopsis mutant, tarani (tni), with enlarged, cup-shaped leaves. Morphometric analyses showed that the positive curvature of the tni leaf is linked to excess growth at the centre compared to the margin. By monitoring the dynamic pattern of CYCLIN D3;2 expression, we show that the shape of the primary arrest front is strongly convex in growing tni leaves, leading to excess mitotic expansion synchronized with excess cell proliferation at the centre. Reduction of cell proliferation and of endogenous gibberellic acid levels rescued the tni phenotype. Genetic interactions demonstrated that TNI maintains leaf flatness independent of TCPs and PEAPODs.
Introduction
The leaf surface is typically flat and is thought to be under genetic control. An early and regulated growth pattern (Kuchen et al., 2012; Remmler and Rolland-Lagan, 2012) is essential for maintaining flatness, and its perturbation can lead to altered surface architecture (Todd, 1985; Coen et al., 2004). For example, if the margin grows more relative to the centre, the leaf surface is expected to buckle to form a saddle-like shape with negative Gaussian curvature (Prusinkiewicz and Barbier de Reuille, 2010), which can be measured as a product of the two principal linear curvatures (Kreyszig, 1991; Nath et al., 2003). Conversely, if the centre grows more relative to the margin, the lamina would form a cup shape with positive curvature. Control of surface curvature has been studied mostly from a biomechanical perspective (Moulia, 2000; Sharon et al., 2002; Klein et al., 2007; Koehl et al., 2008; Liang and Mahadevan, 2009) and little is known about its genetic regulation in leaves, primarily due to the lack of mutants with altered surface curvature, and because the analysis of surface curvature in an expanding organ is difficult (Moulia, 2000; Sharon et al., 2002).
Leaf growth commences with the initiation of the primordium and continues until the leaf reaches its maximum size. An early pattern of growth, which is maintained throughout the growth phase, is established at the primordial stage with maximum growth rate at the base and progressively less towards the tip (Kuchen et al., 2012). Growth is primarily a result of two cellular processes: division and expansion. Based on the mitotic status of the cells, expansion can be of two types: proliferation associated or mitotic expansion and differentiation associated. A newly divided leaf pavement cell measures up to ~100 μm2 in area and expands to approximately double its volume before the next division. This mitotic expansion is characteristically different from differentiation-associated expansion, which takes place when the cell exits the mitotic cycle and enters into terminal differentiation. At this stage, a pavement cell can expand up to ~60 times in area by cell wall loosening and usually does not display mitotic activity under normal circumstances (Donnelly et al., 1999), even though more recent studies predict division of mature pavement cells (Asl et al., 2011). Thus, differentiation-associated expansion plays an important role in leaf growth, whereas proliferation, along with mitotic expansion, provides the organ with more cells.
Leaf kinematic analysis shows that mitotic expansion and differentiation-associated expansion in Arabidopsis leaves are temporally and spatially separated, though with some overlap. Early leaf growth is predominantly contributed by cell division and mitotic expansion, while the later part of growth is primarily due to cell expansion coupled with terminal differentiation (Beemster et al., 2005; Asl et al., 2011). Cell maturity and enlargement are first initiated at the tip of a young leaf and progressively spread towards the base during leaf morphogenesis (Avery, 1933; Donnelly et al., 1999; Nath et al., 2003). Thus, a growing leaf is constituted of larger, differentiated cells at the tip and smaller, proliferating cells at the base, while the interfacing transition region contains cells with both characters. Since increasing number of cells exit the mitotic cycle and enter differentiation, it gives the transition zone an appearance of a moving mitotic arrest front from tip to base, described as the primary arrest front (Nath et al., 2003; White, 2006). A more recent analysis of leaf growth suggests that the size of the proliferative zone, which remains anchored at the lamina–petiole junction, is relatively constant throughout leaf growth, before disappearing rather abruptly (Andriankaja et al., 2012). A second mitotic arrest of the dispersed meristematic cells (DMCs), which give rise to specialized cell types such as stomata and vascular cells, has been proposed (White, 2006). Similar to the primary arrest, DMC arrest is initiated at the tip and seems to progress towards the base as the leaf expands and matures. The DMC arrest is, however, slower than the primary arrest front and is regulated by an independent genetic mechanism.
A large number of genes affecting leaf growth have been isolated and their mutants studied. There are groups of genes that regulate either cell proliferation and mitotic expansion or differentiation-associated expansion (Tsuge et al., 1996; Kim et al., 2003). Among the genes affecting proliferation, there are negative as well as positive regulators. The promoters of cell proliferation include GROWTH-REGULATING FACTORs (GRFs) and GRF-INTERACTING FACTORS (GIFs), AINTEGUMENTA (ANT), NAC1/ATAF2, ARGOS, the vacuolar H+-pyrophophatase AVP1, JAGGED, KLUH, and UBP15 (Mizukami and Fischer, 2000; Horiguchi et al., 2005; Li et al., 2005; Anastasiou et al., 2007; Liu et al., 2008; Gonzalez et al., 2012). Overexpression of these genes increases organ size because of either longer duration or faster rate of cell proliferation and mitotic expansion, whereas loss of function in several of them causes smaller organs made of fewer cells. Some of the factors that suppress cell proliferation are ARF2, PEAPOD (PPD), TCP, DA1/DAR, BIG BROTHER, and ABAP1 (Nath et al., 2003; Palatnik et al., 2003; Okushima et al., 2005; Disch et al., 2006; Schruff et al., 2006; White, 2006; Efroni et al., 2008; Li et al., 2008; Masuda et al., 2008). Loss of function of these genes causes increased organ size and more cell division, and their overexpression can reduce cell number and, consequently, organ size. Compensation between cell division and cell size also plays an important role in controlling organ size (Day and Lawrence, 2000). According to this phenomenon, a decrease in cell number is often partly compensated by a concomitant increase in cell size, and vice versa (Horiguchi et al., 2005; Ferjani et al., 2007).
Even though organ size is altered when expression of these genes is perturbed, leaf flatness is seldom affected. Indeed, mutations in only two groups of homologous genes, CINCINNATA-like TCPs and PEAPODs (PPDs), are reported to influence curvature of the lamina. Mutations in both these genes cause excess cell proliferation and mitotic expansion, leading to larger leaf size, while their gain of function results in smaller leaves composed of fewer cells (Nath et al., 2003; Palatnik et al., 2003; White, 2006; Efroni et al., 2008; Koyama et al., 2010; Sarvepalli and Nath, 2011). However, there are marked differences between the ways these two genes inhibit cell proliferation and mitotic expansion. CIN-like TCP genes regulate the primary arrest of cell division and suppress proliferation-related mitotic expansion more at the margin than in the centre. Consequently, loss of CIN function results in preferential cell proliferation and surface expansion in the margin leading to negative surface curvature. By contrast, PPD genes regulate the arrest of the mitotic growth of the DMCs, and their loss of function results in downward cup-shaped leaves with positive surface curvature.
Studies on genetic control of leaf flatness have been impeded by the limited number of mutants isolated with altered curvature, and because dissection of their geometrical, kinematical, and developmental phenotype is difficult. Consequently, the cellular basis of surface curvature and a genetic framework that regulates this phenomenon has not yet emerged. Here we address this issue by isolating and characterizing a new Arabidopsis mutant, tarani (tni), that produces leaves with upwardly curved, coracle-shaped laminae and by establishing its genetic interactions with other curvature-forming mutants.
Materials and methods
Plant materials and growth conditions
The Arabidopsis thaliana (L.) Heynh. ecotypes Col-0 and Ler were used as wild-type controls. The mutant lines tcp4-2 (GABI_363408), tcp10-2 (SALK_050423), arf2-8 (CS24602), and Δppd (CS16548) were obtained from Stock Centre (http://arabidopsis.org/). The tcp2-1 (SAIL 562-D05) line was a kind gift from Pilar Cubas, Spain. The jaw-D, 35S::ICK2, proCyclin D3;2::GUS, klu-4, and ga1-3 lines have been previously reported (De Veylder et al., 2001; Palatnik et al., 2003; Tyler et al., 2004; Anastasiou et al., 2007; Dewitte et al., 2007). The tni jaw-D, tni jaw-D CyclinD3;2::GUS, tni tcp2 tcp4 tcp10, Δppd jaw-D, and Δppd tcp4 tcp10 lines were genotyped and selected in the F3 generation (list of primers used is given in Supplementary Table S1). EMS-mutagenesis was carried out on Col-0 seeds as described previously (Kim et al., 2006) and the mutants were screened in the F2 generation.
Seeds were sown on plates containing Murashige Skoog medium (catalogue number PT011-1L; Himedia, India) with or without antibiotic, stratified for 3 days in the dark, and moved to a growth room. Seedlings with the first pair of true leaves were transplanted onto soil (Keltech Co., Bengaluru, India). All experiments were performed with plants grown under long-day conditions (16h light/8h dark) at 22°C in a custom-made walk-in Plant Growth Room (Research and Test Equipment Co., Bengaluru, India).
Mapping of tni
The tni mutant in the Col-0 background was crossed with Ler to generate a mapping population, and mapping was carried out as described previously (Jander et al., 2002; Weigel and Glazebrook, 2002). The mutation was mapped to an interval between markers NGA162 and CIW11 on the third chromosome. New CAPS markers TN3C6.4, TN3C6.7, TN3C7.09, and TN3C7.5 were made and the interval was narrowed down to ~0.4Mb, between the TNI3C7.09 and TNI3C7.5 markers (primer sequences are given in Supplementary Table S1). A total of 418 mutant plants were screened. The 0.4Mb interval contained 120 annotated genes.
Leaf measurements
Fifth leaves >4mm long were sandwiched between glass slides and photographed using a Canon Power-shot S90 camera. For leaves <4mm long, photographs were taken after sandwiching on a Wild Heerberg trinocular microscope fitted with a Nikon Coolpix 4500 camera. Cup-shaped leaves of tni and ppd mutants were flattened after introducing incisions at the margin, and care was taken not to include the newly exposed edges while measuring the perimeter (White, 2006). Shape parameters of the crinkly leaves of jaw-D were measured as described previously for cin leaves (Nath et al., 2003). Briefly, crinkly jaw-D leaves were cut into several pieces and each piece was completely spread into a plane between glass slides. Leaf margin was measured for individual pieces, excluding the cut edges, and summed to obtain the perimeter of the whole leaf. In our growth conditions, mature fifth leaves of Col-0, tni, and ppd did not show any serration. Minor serrations observed for jaw-D leaves were ignored and measurement was performed through the middle of the serrations. Measurements of length, width, area, and perimeter were made in the photographs of the flattened leaves or leaf pieces using the straight and free-hand lines tool of the Image J software (rsbweb.nih.gov/ij/). Average ± SEM values are presented in Table 1. The significance of differences was determined by Student’s t-test and the P values are listed in Supplementary Table S2.
Table 1.
Shape and size parameters of the mature fifth leaves of wild-type (Col-0) and mutant plants
| Genotype | Length (mm) | Width (mm) | Perimeter (mm) | Area (mm2) | Length:width ratio | SEM | Perimeter/√Area | SEM | Sample size |
|---|---|---|---|---|---|---|---|---|---|
| Col-0 | 12.8±0.8 | 9.0±0.4 | 36.3±1.6 | 90.3±6.8 | 1.4 | 0.04 | 3.8 | 0.01 | 10 |
| tni | 14.2±1.5 | 16.2±1.4 | 42.7±3.7 | 179.5±28.4 | 0.9 | 0.02 | 3.2 | 0.06 | 9 |
| jaw-D | 14.7±1.8 | 10.3±1.0 | 47.6±4.4 | 118.6±17.2 | 1.4 | 0.05 | 4.4 | 0.10 | 9 |
| tni jaw-D | 15.2±1.8 | 15.1±2.1 | 87.4±16.9 | 267±57.8 | 1.0 | 0.03 | 5.3 | 0.19 | 8 |
| Ler | 12.6±1.3 | 9.6±0.7 | 36.4±3.0 | 93.4±14.0 | 1.3 | 0.04 | 3.8 | 0.03 | 11 |
| ppd | 15.8±1.8 | 9.7±0.8 | 33.4±2.4 | 103.1±19.2 | 1.6 | 0.07 | 3.3 | 0.11 | 5 |
| jaw-D ppd+/– | 15.3±1.7 | 12.5±1.2 | 47.2±4.4 | 145.8±22.6 | 1.2 | 0.03 | 3.9 | 0.0 | 10 |
| 35S::ICK2 (het) | 7.1±1.1 | 5.8±0.9 | 21.7±3.3 | 33.2±10.7 | 1.2 | 0.04 | 3.8 | 0.04 | 6 |
| tni 35S::ICK2 | 10.9±1.9 | 9.8±2.6 | 31.7±5.1 | 84.3±28.2 | 1.1 | 0.12 | 3.5 | 0.15 | 7 |
| ga1-3 | 4.9±0.5 | 3.5±0.6 | 14.0±1.5 | 13.7±3.1 | 1.4 | 0.06 | 3.8 | 0.02 | 15 |
| tni ga1-3 | 6.1±0.7 | 5.6±0.6 | 19.4±2.7 | 27.6±6.9 | 1.0 | 0.06 | 3.7 | 0.03 | 3 |
| Col-0 (–PBZ) | 11.4±1.0 | 8.3±0.6 | 34.5±2.2 | 80.4±4.8 | 1.3 | 0.06 | 3.8 | 0.06 | 6 |
| Col-0 (+PBZ) | 6.3±0.9 | 5.6±0.6 | 18.4±0.9 | 24.3±2.1 | 1.1 | 0.04 | 3.7 | 0.02 | 8 |
| tni (–PBZ) | 14.3±2.1 | 16.1±1.4 | 42.4±4.4 | 176.3±33.2 | 0.9 | 0.03 | 3.2 | 0.05 | 6 |
| tni (+PBZ) | 6.2±0.8 | 6.4±0.8 | 20.8±3.8 | 31.8±6.6 | 1.0 | 0.04 | 3.7 | 0.15 | 11 |
Mean values ± SEM are shown. Statistical significance of difference has been determined by Student’s t-test; P-values are given in Supplementary Table S2.
For calculating the predicted perimeter and area from the measured length and width values at various growth stages, the following standard equations for an ellipse were used (with the assumption that the leaves are all planar ellipses):
and
where P is the perimeter, A is area, a is half of the length and b is half of the width.
For the leaf growth rate experiment, the width of a given leaf of Col-0 and tni was first measured at emergence (<1mm long), and measurements were then made on alternate days for the next 20 days. The width of leaves >3mm long was measured using non-elastic stitching thread, and the absolute width was calculated using a ruler. The width of leaves <3mm long was measured using a thin copper wire with minimum graduation of 250 μm [graduation was made manually using a Rabone scale (UK)].
Epidermal cell measurements
For measuring epidermal cell size, impressions of adaxial and abaxial surfaces of leaves were taken with dental wax (PRESIDENT, article no. 4667, Coltene, Switzerland). Casts were made with Araldite (Huntsman Advanced Materials Pvt. Ltd, India), using the dental wax on the moulds, and gold-coated with sputter coater (Jeol, Germany); these were loaded on to the stage of an ESEM Quanta 200 scanning electron microscope (Fei Company, USA). Images were taken at 25kV with a spot size of 3.0nm (the spot size is the space occupied by the cone of the electron beam on the sample surface) and working distance of 10mm. The average cell size per field was calculated by dividing field area by number of cells. A minimum eight fields were averaged to obtain the cell size in a leaf. Trichomes were photographed using a light microscope. For determining the frequency of trichome branching, mature, adaxial trichomes were observed with the light microscope, the number of branches was noted, and the frequency was expressed as a percentage of the total number of trichomes.
Gus assay
Gus assay was performed as described previously (Sessions et al., 1999). Samples were collected in 90% acetone on ice, incubated at room temperature for 30min and washed with staining buffer (50mM sodium phosphate pH 7.0, 0.2% Triton X-100, 10mM potassium ferrocyanide, 10mM potassium ferricyanide). Fresh staining buffer was added along with 1mM X-Gluc and vacuum infiltrated for 30min followed by incubation at 37°C for a maximum 90min. Staining buffer was replaced with 70% ethanol and this was incubated for 2h at room temperature; samples were then transferred to Hoyer’s medium (Anderson, 1954) and incubated for 1 day for clearing, following by mounting on glass slides. Observation and image acquisition were performed using an Olympus BX51 trinocular microscope (Olympus, Japan) fitted with ProgRes C3 camera using ProgRes Capture Pro 2.6 software. Multiple high resolution photographs were taken and merged in Adobe Photoshop CS3 to obtain panoramic view images of the entire leaf. For measuring the rate of progression of the mitotic arrest front in the distal-to-proximal direction, the entire leaf length and relative position of the arrest front were measured using Image J software.
For measuring the shape of the arrest front, equidistant, rectangular fields of 200×100 μm were demarcated within the mitotic arrest front from midrib to margin. Initially, in each field, the total numbers of epidermal and GUS-producing cells were manually counted, and the percentage of GUS-producing cells was calculated (Supplementary Figure S1A). For more objective measures, all the pictures were then run by iLASTIK, an open source image segmentation tool, to classify the pixels based on intensity (Sommer et al., 2011). Segmentation of GUS-positive cells as one class and the rest as a background class was done based on selected features such as colour, edge, and texture (Supplementary Figure S1B). The images were then processed using CELL PROFILER software, with classification metadata input from iLASTIK, to identify GUS-positive cells (Jones et al., 2009). In each field, total numbers of epidermal and GUS-producing cells were counted and the percentage of GUS-producing cells was calculated. The data analysed by the manual method and image segmentation tool produced similar results (Supplementary Figure S1C). When the measurements were carried out, the mitotic arrest front was at ~30–40% of the leaf length from the tip. It is important to note that the mitotic arrest front is not a sharp boundary. However, the shape of the arrest front did not change when measurements were taken at slightly different positions along the proximal-to-distal axis within the arrest front. Serrations were usually avoided while recording the measurements.
Treatment of plants with paclobutrazol
For whole plant application, plants were treated with 120 µM paclobutrazol (PBZ; Duchefa Biochemie, The Netherlands) as described previously (Jacobsen and Olszewski, 1993). For treatment of a single leaf, 120 µM PBZ was applied on the fifth rosette leaf using a paint brush (Camlin, India; No. 00 with three-quarters of the bristles removed). Application started with the emergence of the leaf (~1mm long) and was continued 2–3 more times on alternate days. Mature leaves of untreated and treated plants were photographed and morphometric parameters were measured.
DNA microarray experiments
Total RNA was isolated from 1–3mm long rosette leaves on the fifth node using the RNeasy Plant Mini Kit (Qiagen, Germany) from two different plant populations for biological replicates. The RNA samples were labelled with a single colour (Cy3) and hybridized on a 4×44 K Arabidopsis Agilent microarray chip (G2519F_021169); the intensity of spots was measured and normalized according to company specifications (Agilent Technologies, USA). The signal intensities of replicates were averaged and compared. The list of genes affected has been submitted to Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE38111. For a comparison of the tni gene expression profile with those under gibberellic acid (GA) treatment or with tcp mutants (Schommer et al., 2008; Ribeiro et al., 2012), publicly available expression data sets were obtained and analysed using Genevestigator tools (https://www.genevestigator.com).
Results
tni leaves produce larger, cup-shaped lamina
The tarani (tni; ‘boat’ in Sanskrit) mutant was identified in a forward genetic screen aimed at isolating mutants with altered leaf shape. The recessive tni mutation mapped between 7.1 and 7.5Mb of chromosome 3. A mature tni leaf is larger, rounder, and curves upwards to form a cup-shaped lamina, while wild-type Arabidopsis leaves are flat with an ovoid shape (Fig. 1). To study the effect of the tni mutation on leaf shape, we measured the length and width of mature tni leaves and compared these with wild-type values. While Col-0 leaves on the fifth node from the base showed a length:width ratio of 1.4, tni leaves grew slightly more in length but almost twice as much in width, reducing the final length:width ratio to 0.9 (Table 1). This reduced leaf index value was established when the leaves were <1mm long (Fig. 2A), suggesting that the growth defect in the tni lamina is established early in development. Increased growth also resulted in a significantly larger tni leaf. While the average area of a mature Col-0 leaf was 90.3mm2, a mature tni leaf grew up to 180mm2 (Table 1). This excess growth resulted from a faster growth rate as well as longer length of growth in the medio-lateral direction, as measured by the change in width over time (Fig. 2B). At the fastest growth phase, the tni leaves grew at a rate twice that of the wild-type value of 0.5mm day–1. Growth kinetics of tni leaves along the length axis, however, were comparable to those of the wild type (Supplementary Figure S2).
Fig. 1.
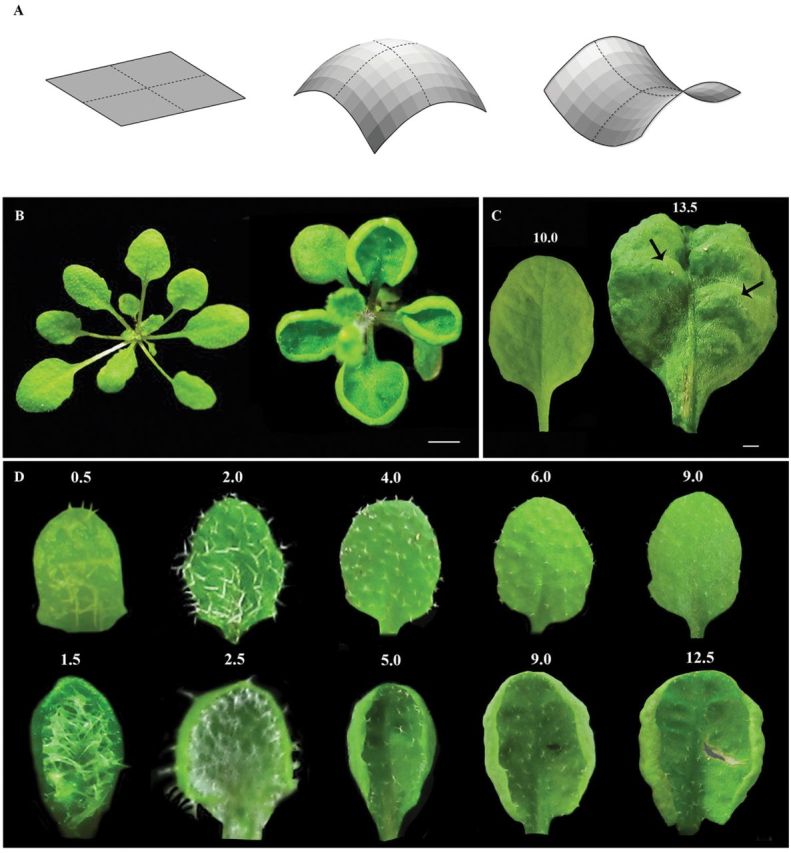
Phenotype of tni leaves. (A) Schematic representation of surfaces with zero (left), positive (middle), and negative (right) Gaussian curvature, which is measured by the product of linear curvatures along orthogonal axes (dotted lines). (B) 1-month-old Col-0 (left) and tni (right) rosettes showing differences in leaf shape. Scale bar = 1cm. (C) Abaxial view of mature Col-0 (left) and tni (right) leaves. Bulges in the tni lamina are indicated by arrows. Numbers indicate leaf length in mm. Scale bar = 5mm. (D) Adaxial views of Col-0 (top) and tni (bottom) leaves at various growth stages, highlighting differences in shape and curvature. Note that positive curvature in the tni lamina is already visible in leaves of 1.5mm length. Numbers above the leaves indicate leaf length in mm.
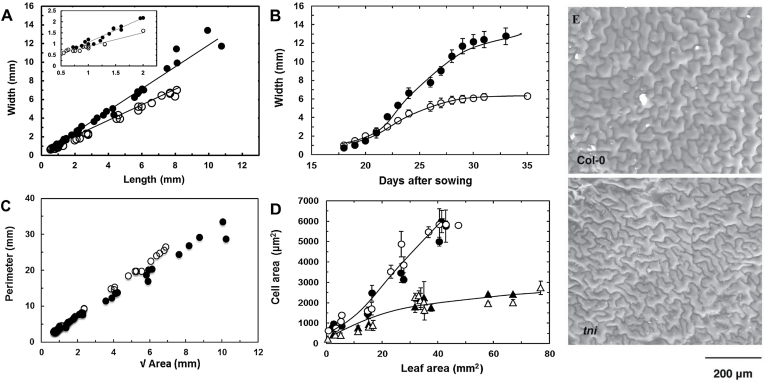
Fig. 2.
Morphometric analyses of the tni leaf. (A) Width and length of growing Col-0 (open circles) and tni (filled circles) leaves. The inset highlights shape differences at an early growth stage. (B) Growth kinetics of Col-0 (open circles) and tni (filled circles) leaves. (C) Leaf perimeter values are plotted against √Area for Col-0 (open circles) and tni (filled circles) leaves. When straight lines are fitted through the data points (not shown), the slopes (P/√A) are 3.72 for Col-0 and 3.07 for tni. (D) Average cell size of adaxial (open circles, open triangles) and abaxial (filled circles, filled triangles) surfaces of Col-0 (open circles, filled circles) and tni (open triangles, filled triangles) leaves. Error bars indicate SEM (n = 8–10 leaves). (E) Electron micrographs of adaxial epidermal cells of mature leaves.
Unlike Col-0 leaves, the cup-shaped tni leaves could not be flattened without introducing cuts in the margin (Supplementary Figure S3). A surface can in principle be flat or curved. The Gaussian curvature (Fig. 1A; Prusinkiewicz and Barbier de Reuille, 2010) provides a curvature measure, which is tightly related to lateral surface expansion. Young tni leaves form a cup-shaped lamina (Fig. 1D) with positive Gaussian surface curvature. At later stages of growth, the leaf margin folds back onto itself (Fig. 1B, D) making the surface curvature heterogeneous across the lamina. Since measuring the Gaussian curvature of such a surface is difficult, we used a proxy measurement of surface curvature by dividing the perimeter (P) with the square root of the area (√A). We are aware that this parameter (P/√A) suffers from inaccuracy since its value is dependent on the distribution of the Gaussian curvature across the surface, as well as on the shape in the planar dimension. Nevertheless, in the absence of a more accurate parameter, P/√A has been used prevously as a measure of leaf surface curvature (Nath et al., 2003; White, 2006).
The values of the length:width ratio show that the tni leaves are less elliptical than the Col-0 leaves (Table 1). In order to determine to what extent this difference in planar shape would contribute to their P/√A values, we first calculated their theoretical P/√A values from the measured leaf length and width (see Materials and Methods). Both tni and Col-0 leaves showed similar values of ~3.6 (Supplementary Figure S4), suggesting that the difference in their planar shape has little effect on the P/√A parameter. We then determined the actual P/√A values from direct measurements of perimeter and area (Fig. 2C, Table 1; Supplementary Figure S4) and compared these between the two genotypes. Mature Col-0 leaves have flat laminae with an average perimeter of 36.3±1.6mm and a P/√A value of 3.8±0.3 (Table 1). The measured P/√A values of Col-0 were slightly higher than the predicted values at all growth stages (Supplementary Figure S4), perhaps due to an imperfect elliptical shape of wild-type leaves. Even though the average perimeter of mature tni leaves increased to 42.7±3.7mm, the P/√A value was reduced to 3.2±0.3, possibly due to excess medial growth compared to the margin. The P/√A of the Col-0 leaf was maintained at a constant value throughout the growth phase (Fig. 2C, Supplementary Figure S4)). However, although the P/√A value of the early tni leaf was comparable with the Col-0 value, it decreased progressively during later growth stages, suggesting that excess medial growth in the tni leaf continued throughout leaf morphogenesis.
Cell proliferation is prolonged in tni leaves
To examine the basis of excess growth, we compared the epidermal cell size of tni leaves with that of Col-0 (Fig. 2D, E). The pavement cells of mature Col-0 leaves on the fifth node from the base grew to ~6000 µm2 while the tni pavement cells grew to ~3000 µm2. Thus, even though tni leaves are larger, they are made of more, smaller cells, suggesting excess cell proliferation. While an average mature Col-0 leaf contains ~15 000 pavement cells, a tni leaf had about four times more cells. As in Col-0, the tni pavement cells at the adaxial surface were of similar size to those at the abaxial surface at all growth phases (Fig. 2D). The frequency distribution of size of adaxial and abaxial epidermal cells, sampled throughout the surface of mature Col-0 and tni leaves, showed a similar trend (Supplementary Figure S5), i.e. in both Col-0 and tni leaves the smaller cells are more abundant in the abaxial surface whereas the larger cells are more abundant in the adaxial surface. Thus, it is plausible that the upward curvature of tni leaves does not arise from differential expansion of cells on the two surfaces. However, it should be kept in mind that a minor difference in cell size or cell proliferation rate between the adaxial and abaxial epidermal cells can cause upward bending of leaves. Nevertheless, when cell proliferation and mitotic expansion were reduced by overexpressing the cell division inhibitor ICK2 (De Veylder et al., 2001), the tni leaves became smaller with flat laminae (Table 1, Fig. 5), highlighting the role of cell proliferation in the tni phenotype.
Fig. 5.
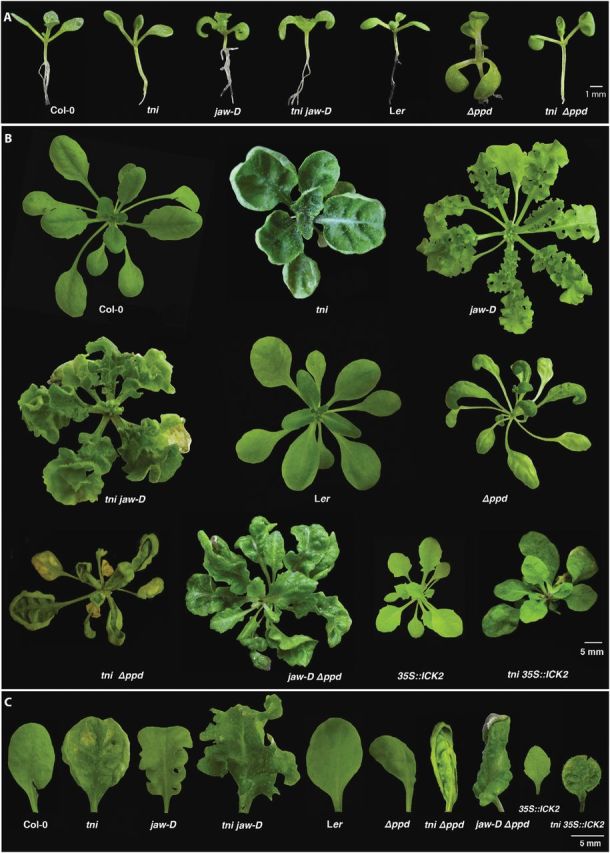
Genetic interactions of tni with other curvature mutants. (A) 12-day-old seedlings of the genotypes indicated highlighting the cotyledon epinasty phenotype and its rescue. (B) 25–40-day-old rosettes of the genotypes indicated. The 35S::ICK2 plant was heterozygous for the transgene. The tni 35S::ICK2 individual had at least one copy of the 35S::ICK2 transgene. (C) Mature leaves on the fifth node of the genotypes indicated are shown.
Given that the proliferation zone remains anchored at the leaf base throughout the growth phase (Andriankaja et al., 2012), its size and the dynamics of cell exit from this zone are likely to influence leaf shape and size (Powell and Lenhard, 2012). CIN-like TCP genes accelerate leaf maturation, possibly by restricting the mitotic zone and/or promoting cell exit from this zone (Nath et al., 2003; Efroni et al., 2008). To examine the dynamics of cell proliferation in tni leaves, we measured the cell division pattern in young Col-0 and tni leaves expressing the GUS reporter under the transcriptional regulation of CYCLIN D3;2, a gene encoding a mitotic cyclin required for developmentally regulated cell division (Dewitte et al., 2007). In a 0.9mm long Col-0 leaf, transcription of CYCLIN D3;2 was observed throughout the lamina except at the distal region and midrib (Fig. 3). Expression was progressively restricted towards the base as the leaf grew, and little expression was observed at the base of a ~3mm long leaf. A mitotic arrest zone was also observed at the tip of a young tni leaf, which expanded more slowly than in Col-0 (Fig. 3). Reduced mitotic activity was also observed at the mid-vein and near the vasculature of tni leaves.
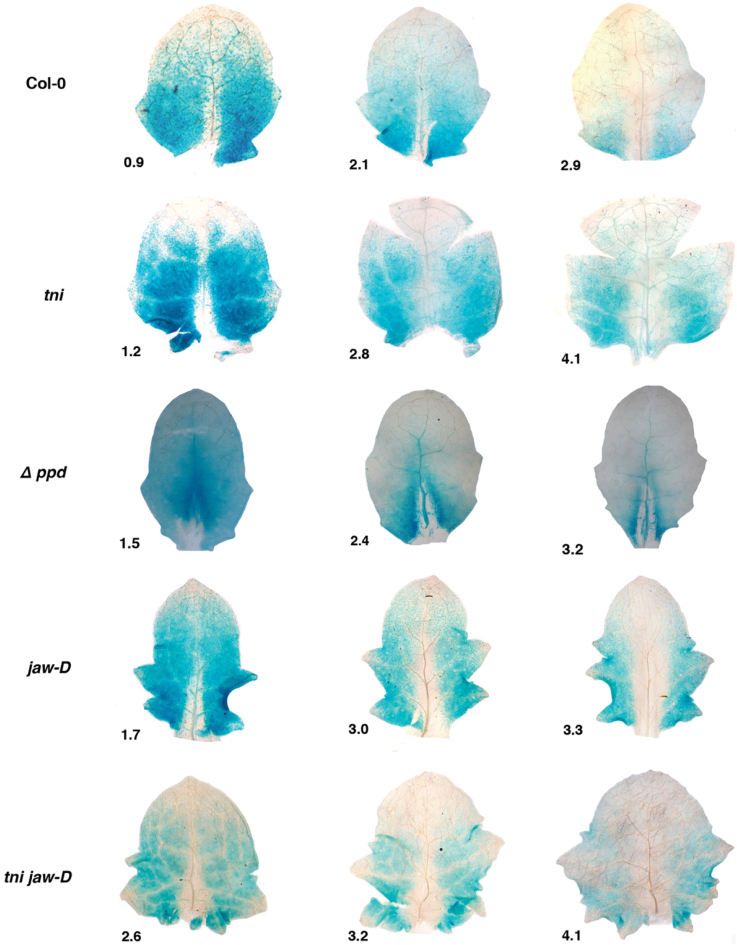
Fig. 3.
Cell proliferation activity in young leaves. CYCLIN D3;2 expression is shown as GUS reporter activity (blue dots) in young leaves of the genotypes indicated. Numbers denote leaf length in mm.
To compare the dynamics of cell proliferation in tni and Col-0 leaves, we measured the length of the proliferation zone proximal to the arrest front. In a developing leaf, the transition from the distal arrested zone and the proximal proliferating zone is gradual and no boundary can be drawn between the two (Fig. 3) (Nath et al., 2003; Efroni et al., 2008; Andriankaja et al., 2012). Therefore, the proliferation zone was arbitrarily defined as the distance between the base of the lamina and the transition zone where ~10% of adaxial cells showed GUS activity adjacent to the midrib. In Col-0, for every 1mm growth in total leaf length, the proliferation zone decreased by 0.37mm (Fig. 4A). In contrast, the tni proliferation zone first increased by 0.38mm for every 1mm growth in total leaf length and then sharply decreased (data not shown).
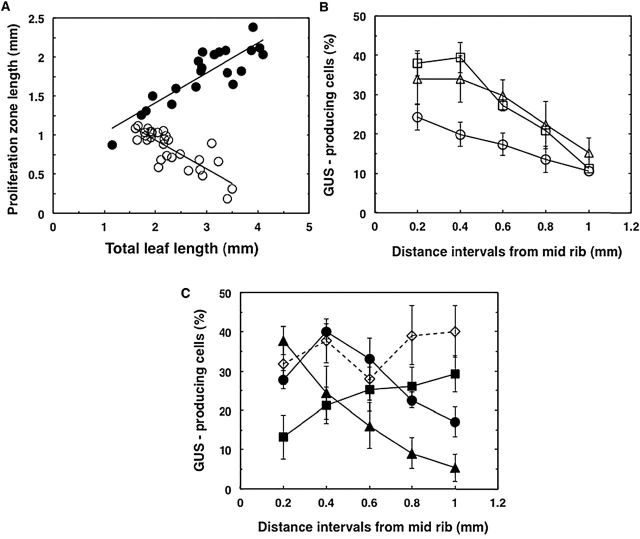
Fig. 4.
Cell proliferation dynamics along the orthogonal axes of young leaves. (A) Length of proliferation zone plotted against total length of Col-0 (open circles) and tni (filled circles) leaves. Each data point represents a measurement from a single leaf. A straight line was fitted to the data points (R 2 is 0.67 for Col-0 and 0.76 for tni) and the slope values for Col-0 and tni were –0.37 and +0.43mm–1, respectively. (B, C) Percentage of GUS-producing cells in the transition zone (refer to Supplementary Figure S1) is plotted against distance from midrib to margin of Col-0 (open circles), arf2-8 (open squares), and klu-4 (open triangles) (in B) and of tni (filled circles), jaw-D (filled squares), Δppd (filled triangles), and tni jaw-D (open diamonds) (in C). 100–150 pavement cells were counted per data point per region of leaf. Mean values of 11 leaves in Col-0 and five leaves for other genotypes are shown. Leaf length varied from 1.7 to 2.2mm (Col-0 and klu-4), 2.3 to 2.8mm (tni), 2.8 to 3.3mm (jaw-D), 1.9 to 2.6mm (Δppd and arf2-8), and 3.2 to 4.0mm (tni jaw-D). The mitotic arrest front was at ~one-third of the leaf length from the tip when the analysis was carried out. Error bars are SEM.
Cell proliferation pattern along the medio-lateral axis is altered in tni leaves
In young cin leaves, the shape of the mitotic arrest front within the transition zone is strongly concave, leading to more growth in the margin and crinkly laminae. One may then predict that changing the shape of the arrest front to highly convex would lead to excess growth in the centre compared to the margin, resulting in a cup-shaped leaf (Sarvepalli and Nath, 2011). To examine whether the shape of the arrest front is changed in the tni leaf compared to Col-0, we compared cell proliferation between the medial region and margin. We estimated the mitotic density by measuring the percentage of GUS-producing cells, which reports CYCLIN D3;2 expression, along the medio-lateral axis within the transition zone and expressed it as a function of distance from the midrib. In young Col-0 leaves, ~25% of cells near the midrib expressed GUS and the value progressively decreased to 10% near the margin (Fig. 4B, Supplementary Figure S1C), giving a mild convex shape to the mitotic arrest front, as was observed in young Antirrhinum leaves (Nath et al., 2003). The mitotic arrest front showed similar overall shape when measured at various places within the broad transition zone (data not shown). A progressive decrease in mitotic cell density from the medial region to the margin was also observed in the flat leaves of mutant lines where leaf size was either increased (auxin response factor2-8) or decreased (klu-4) (Fig. 4B; Supplementary Figures S6, S7; Schruff et al., 2006; Anastasiou et al., 2007), suggesting that perturbed cell division per se does not lead to a change in the shape of the mitotic arrest front.
In young tni leaves, ~27% of cells produced the GUS reporter near the midrib within the mitotic arrest front. However, the proportion of the GUS-producing cells increased to ~40% slightly towards the margin and then decreased to ~15% at the extreme margin (Fig. 4C), giving the arrest front a strong convex shape with a dip at the midrib. The Δppd leaves which produce downward-curving, cup-shaped lamina (White, 2006) also showed a strong convex-shaped arrest front (Figs. 3, 4C). A strongly convex-shaped mitotic arrest front is expected to result in excess mature cells around the medial region compared with the margin, leading to cup-shaped leaves. In contrast to tni and Δppd leaves, the arrest front in the jaw-D leaf was concave, which might lead to more cell proliferation and mitotic expansion in the margin than the centre, consistent with its negative surface curvature (Figs. 3, 4C). These results possibly imply a link between varying cell division patterns along the medio-lateral axis and curvature differences in diverse genetic backgrounds.
TNI maintains leaf flatness independent of CIN-like TCP genes and PEAPODs
When five CIN-like TCP genes were downregulated by overexpressing the micro RNA MIR319 in the jaw-D line, or by combining all the loss-of-function mutations, the resultant plants showed a crinkled leaf margin (Palatnik et al., 2003; Schommer et al., 2008; Koyama et al., 2010). Differentiation was delayed in these leaves and the progression of the mitotic arrest front was slowed (Efroni et al., 2008). To determine whether TNI controls leaf flatness independent of TCP and PPD genes, we generated mutant combinations by crossing tni with jaw-D, tcp2 tcp4 tcp10, and Δppd and studied their shape parameters (Figs. 5, 6; Table 1). The jaw-D leaves showed a slight increase in both length and width compared to the wild type, without altering the leaf index value. The length of jaw-D was also comparable to that of the tni jaw-D double mutant, suggesting a minor role played by these two genes affecting growth in the proximo-distal axis. In contrast, the width of the jaw-D leaf was increased by >60% in the tni mutant background, suggesting that TNI controls growth in the medio-lateral axis independent of the TCP genes. Consequently, the leaf index value of the tni jaw-D line was reduced to 1.01, much less than the jaw-D value.
Although the perimeter and area of jaw-D leaves increased by ~30% compared to Col-0, P/√A increased to 4.3 compared with the Col-0 value of 3.8 (Table 1), possibly indicating excess margin growth (Nath et al., 2003). In the tni jaw-D leaves, width, perimeter, and area exceeded the values of the single mutants. The tni jaw-D leaves were more crinkly than the jaw-D single mutant, with a net surface curvature value of 5.3, possibly due to excess growth (Nath et al., 2003). The shape of the mitotic arrest front of young tni jaw-D leaves appears to show a combination of those in respective single mutants (Figs. 3, 4C), possibly implying that TNI and TCP genes act independently. The tni tcp2 tcp4 tcp10 quadruple mutant also showed phenotypic features similar to the tni jaw-D mutant (Fig. 6), demonstrating that the tni jaw-D phenotype was indeed due to combined loss of TNI and TCP function and not due to a TCP-independent overexpression effect of miR319 (Palatnik et al., 2003). The tni Δppd double mutant also showed features of both tni and Δppd laminae, with a combination of up- and downward folds (Fig. 5), suggesting an independent function of these two genes.
Fig. 6.

Genetic interactions among surface curvature mutants. 30–35-day-old rosettes of the genotypes indicated are shown. Note that the highly crinkly phenotype of jaw-D leaves is rescued to almost flat in the Δppd/+ background.
When the Δppd mutant was crossed with jaw-D, the resultant jaw-D Δppd double homozygous plants produced leaves with phenotypes that were intermediate between the single mutants (Fig. 5). Interestingly, Δppd/+ completely rescued the crinkliness of the jaw-D leaves and produced flat Δppd/+ jaw-D leaves that resembled Col-0 (Fig. 6; Table 1), reiterating the dominant nature of the Δppd mutation (White, 2006). When Δppd was crossed with the tcp4 tcp10 double mutant, which produces mildly crinkly leaves, the resultant triple mutant (Δppd tcp4 tcp10) also produced leaves with features of both parental lines (Fig. 6). Taken together, these results show that the TNI, TCP, and PPD genes maintain leaf flatness independent of one another.
Reduction of endogenous GA levels rescue the tni phenotype
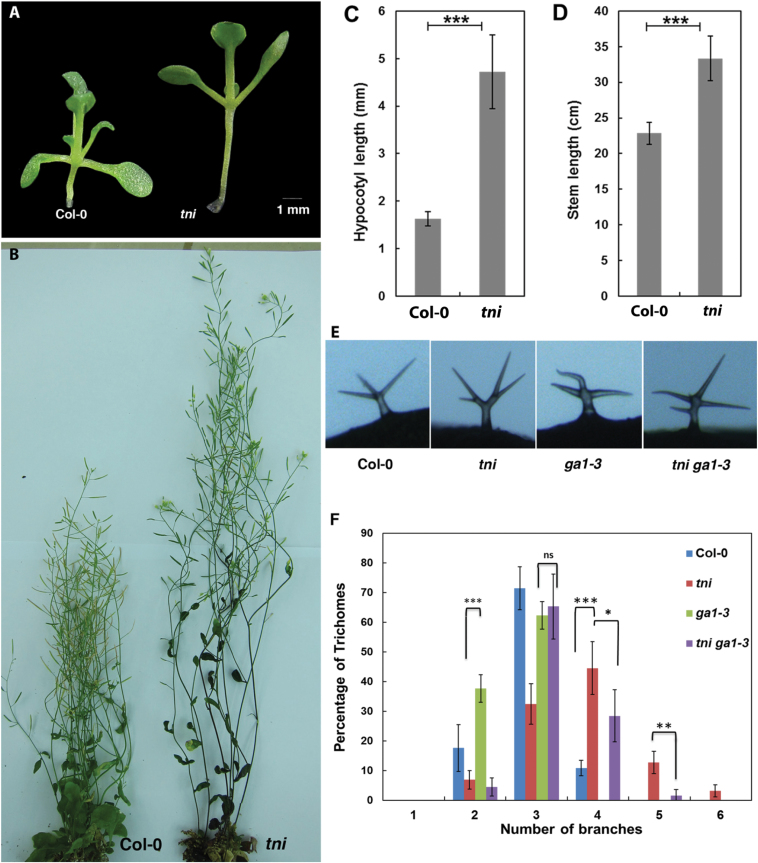
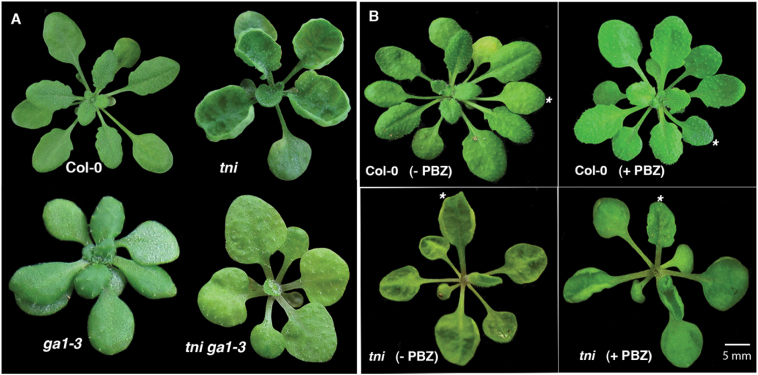
Distribution of endogenous GA in a young leaf is closely associated with leaf morphogenesis in diverse plant species (Achard et al., 2009; Nelissen et al., 2012). GA regulates several aspects of Arabidopsis development, including cell proliferation, expansion, endoreduplication-mediated trichome branching, and flowering (Wilson et al., 1992; Chien and Sussex, 1996; Jacobsen et al., 1996), consistent with its ubiquitous distribution and activity (Silverstone et al., 1997; Tyler et al., 2004). The tni plants display features resembling elevated GA levels/signalling, such as increased length of the hypocotyl and stem, fewer rosette leaves, and hyper-branched trichomes (Fig. 7; Supplementary Figure S8; Silverstone et al., 2007). To determine whether tni leaf shape is associated with altered GA levels, we reduced its endogenous GA level by crossing it with the GA-deficient mutant ga1-3 (Sun et al., 1992). The ga1-3 plants produce smaller leaves with flat laminae. The tni ga1-3 double mutant also produced flat leaves (Fig. 8A), although leaf area decreased compared to tni (Table 1). The rescue of tni leaf curvature by the ga1-3 mutation was not due to a change in leaf size, since curvature rescue was not observed when leaf size was either reduced or increased independently in the tni klu-4 or tni arf2-8 double mutants, respectively (Supplementary Figure S9). The ga1-3 mutant also rescued other tni phenotypes, including trichome hyper-branching (Fig. 7E, F). Rescue of tni curvature was independently achieved by external application of the GA antagonist paclobutrazol (PBZ). Both systemic (Supplementary Figure S10) and local (Fig. 8B) applications of PBZ on young tni leaves resulted in rescue of positive curvature, with the P/√A value restored to its Col-0 level (Table 1). Taken together, reduction of endogenous GA levels partly rescued the tni leaf phenotype.
Fig. 7.
GA-related phenotypes of the tni mutant. (A, B) Seedlings from the root–shoot junction and above (A) and mature plants (B) of Col-0 and tni are shown, highlighting the elongated hypocotyl and stem in tni. (C, D) Plots of hypocotyl length (C) averaged over 29 seedlings each in Col-0 and tni, and stem length (D) averaged over 13 seedlings each in Col-0 and tni. Error bars represent SEM. ***, P ≤ 0.001. Student’s t-test was used. (E) Microscope photographs of representative trichomes of the genotypes indicated highlighting altered branching phenotypes. (F) Frequency of trichomes (as a percentage of the total) of genotypes indicated plotted as a function of trichome branch numbers. 297–712 trichomes from 3–6 mature leaves were scored for their branch numbers and plotted. Error bars represent SEM. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05. Student’s t-test was used.
Fig. 8.
Reduction in GA level rescues the tni leaf phenotype. (A) Rosettes of the genotypes indicated are shown. Note the rescue of tni leaf curvature in the ga1-3 background. (B) 1-month-old rosettes of Col-0 and tni plants with (+PBZ) or without (–PBZ) PBZ treatment on the fifth leaf (*). Note the rescue of positive curvature only in the fifth tni leaf.
Global transcriptome analysis of young tni leaves
In the absence of TNI identity, we attempted to gain insight into TNI function by comparing the global transcripts of young tni leaves with those of Col-0 by DNA microarray analysis. A total of 770 genes were upregulated and 944 genes downregulated by ≥2-fold in tni leaves (Supplementary Figure S11). The genes that were significantly upregulated in tni leaves were involved with the cell cycle, proteolysis, RNA processing, and transposons. Eighteen cell cycle-related genes were upregulated in the tni mutant, which did not include cyclins or cyclin-dependent kinases. Genes preferentially downregulated in the tni mutant were involved with signalling processes, cell wall modification, general metabolism, stress, and phytohormones.
Since the tni phenotype resembled an elevated GA phenotype in several aspects (Fig. 7), we compared the transcripts differentially regulated in tni leaves with those in GA-treated seedlings that are available in the public domain (Ribeiro et al., 2012). We did not observe any significant similarity between the overall transcript changes between tni and GA-treated plants (Supplementary Table S3). While expression of 125 genes changed in a similar way in tni and GA-treated plants, expression of 214 genes showed opposite expression changes in tni and GA-treated leaves. We also compared the tni transcriptome profile with that in jaw-D and TCP gain-of-function lines, since cell proliferation is deregulated in both tni and tcp leaves (Efroni et al., 2008). Similarly, tni microarray data was compared to that of TCP mutants (tcp2;tcp4) and rTCP4 (Schommer et al., 2008); little similarity was found between the transcriptome profiles.
Discussion
Leaf growth is a result of cellular expansion during cell proliferation and differentiation. Genes that promote proliferation are active throughout the lamina at a very early growth stage (Horiguchi et al., 2005; Rodriguez et al., 2010) and produce adequate numbers of cells. Recent leaf growth models show that the zone of proliferation remains anchored at the base and its size stays more or less constant throughout the growth phase (Andriankaja et al., 2012). The newly divided cells are pushed away from the proliferation zone towards the leaf tip to form an arrest zone, where they lose their proliferation potential and enter into a phase of differentiation-associated expansion. As more and more cells enter the differentiation phase, the arrest zone increases in size, reducing the relative proportion of the proliferation zone. The proliferation zone then disappears rapidly, terminating cell division and eventually leaf growth (Andriankaja et al., 2012). Several factors, such as the size of the proliferation zone and the duration of its activity, the rate of cell exit into the arrest zone, and the rate of cell division and expansion, are likely to have profound effect on the relative area occupied by these two regions, and consequently on the final leaf size (Gonzalez et al., 2012; Powell and Lenhard, 2012). A premature termination of the proliferation zone would result in a smaller leaf with fewer cells, whereas its prolonged presence would lead to a bigger leaf with excess cells (Mizukami and Fischer, 2000; Nath et al., 2003; White, 2006; Efroni et al., 2008; Sarvepalli and Nath, 2011). The TNI gene possibly restricts the size as well as the duration of the proliferation zone (Figs. 3, 4A) and thereby acts as a negative regulator of leaf growth. A smaller arrest zone during early growth of tni leaves may arise from reduced cell expansion (Fig. 2D, E) and fewer cells exiting the proliferation zone.
Kinematic analysis of root (Beemster and Baskin, 1998; Beemster et al., 2002; Silk, 2006) and leaf growth (De Veylder et al., 2001; Beemster et al., 2005; Asl et al., 2011) in wild-type Arabidopsis has uncovered the relationship between organ growth and the division/expansion of cells. Our analysis, however, does not clarify the exact role of TNI on cell division and expansion. A larger proliferation zone in tni leaves may result from increased proliferation-promoting mobile signal at the leaf blade/petiole junction (Kazama et al., 2010), elevated cell division rate, or a reduction in the rate of cell exit into the arrest zone. In Arabidopsis, the duration of the cell cycle remains relatively constant throughout leaf development (Asl et al., 2011). In most mutant leaves with increased cell proliferation, such as cin, jaw-D, and 35S::ANT, the duration of proliferation is increased with no significant change in cell cycle rate (Mizukami and Fischer, 2000; Nath et al., 2003; Efroni et al., 2008). By contrast, leaf growth rate as well as duration increased in tni leaves (Fig. 2B). Whether TNI affects the rates of cell division and cell expansion can be resolved only by direct measurements using time-lapse microscopy.
Surface curvature in the leaf is expected to be influenced by regulated growth between the margin and medial regions. There are no significant margin-to-medial differences in cell division between the distal- arrested zone and the proximal division zone. However, the interfacing transition region might play an important role in maintaining leaf flatness. Within the transition region of the wild-type leaf, cell division in the medial region is slightly higher than at the margin, giving the so-called mitotic arrest front a slightly convex shape (Fig. 4B) (Nath et al., 2003). In the tni leaf, differences in cell division and associated cell expansion between the medial and margin of the transition zone become stronger. If cell expansion is not differentially affected between these two regions due to compensation, this may result in excess medial growth and possibly in positive Gaussian curvature. TNI-mediated control of differential cell proliferation is independent of TCP genes (Figs. 4C, 5), mutation in which causes excess cell division at the margin. TCP-mediated suppression of marginal division is perhaps stronger than TNI-mediated suppression of cell division at the medial region, leading to a resultant negative Gaussian curvature in the tni jaw-D double mutant leaf (Fig. 5B, Table 1).
It is interesting to note that even though both tni and Δppd leaves make cup-shaped laminae, the former produce upwardly curving leaves while the latter make downwardly curving leaves. While the reason for this difference is not apparent from the data presented here, it is possible that small differences in cell proliferation or expansion rates between the adaxial and abaxial surfaces bring about the opposite effects. Detailed kinematic studies of cell division/expansion in these mutant leaves may uncover this difference. Another reason may be that the PPD genes control the proliferation of dispersed meristemoid cells (White, 2006), the density of which may vary between the adaxial and abxial surfaces. The role of TNI on meristemoid cell proliferation has not been observed.
From the analysis of mutants in genes involved in leaf morphogenesis, it is becoming increasingly clear that the control of cessation of cell proliferation, and the transition from mitotic to differentiation-associated expansion, is of prime importance in maintaining leaf shape and size. Marked effects on size and shape are observed when genes that restrict cell proliferation, such as TCP, TNI, and PPD, are mutated. Downregulation of TCP and TNI genes produces leaves with larger area, while their overactivation drastically reduces leaf size and cell number (Koyama et al., 2010; Sarvepalli and Nath, 2011). More dramatic effects could be observed when two genes were mutated together in combinations of double mutants (Figs. 5, 6; Table 1), suggesting that these genes suppress leaf cell proliferation independently of one another. Their domains of action appear to be complementary as well; while TCP genes suppress cell proliferation and mitotic expansion primarily at the margin, TNI does so more at the centre (Figs. 5, 6), and consequently their mutations cause opposite leaf curvatures. Leaf flatness possibly depends on a delicate balance of the activities of these genes. Loss of both TNI and TCP genes does not result in a flat leaf, possibly due to a stronger deregulation of cell proliferation and mitotic expansion at the margin by the jaw-D mutation than that at the centre by the tni mutation.
Heterochronic factors such as CIN-like TCP genes advance the timing of the transition from proliferation to expansion (Nath et al., 2003; Efroni et al., 2008; Sarvepalli and Nath, 2011). Growth continues for a longer duration in the tni leaves as well (Fig. 2), presumably due to a more persistent and wider cell proliferation zone at the leaf base (Fig. 3, 4C). In this regard, TNI acts as a heterochronic gene in leaf maturation. However, faster growth of the tni leaf suggests a more direct role of TNI in cell proliferation and mitotic expansion (Figs. 2B, 3), which could result from elevated cyclin function. Increased growth rate has been reported in plants overexpressing CYCLIN D2 (Cockcroft et al., 2000). Interestingly, excess cell division in the tni mutant results in differential growth in two orthogonal axes: more in the medio-lateral axis than in proximo-distal axis (Table 1). It will be interesting to examine whether this polar preference in cell division and anisotropy of mitotic expansion is related to a change in the orientation of the plane of cell division.
The identity of the TNI gene is not yet known. The effect of the tni mutation on cell proliferation suggests that TNI encodes a cell division inhibitor that reduces the rate of the cell cycle in a spatially regulated manner. Faster growth rate and prolonged CYCLIN D3;2 expression is observed in young tni leaves. Further, a general reduction of cell proliferation by overexpressing the cell division inhibitor ICK2 partly rescued the tni phenotype. Super-numerary branches in tni trichomes (Figs. 7E, F) also indicate elevated CYCLIN function in the mutant, since overexpression of B and D cyclins can increase trichome branching via elevated endoreduplication (Ishida et al., 2008). However, we did not observe an increased level of any CYCLIN or CDK transcripts in the tni leaves by global transcript analysis. Therefore, it is possible that TNI restricts cell division not by transcriptional control of the cell cycle factors, but through post-translational modification or degradation of the target proteins. The link between TNI function and the cell cycle could also be more indirect and through plant hormones (Koyama et al., 2010; Yanai et al., 2011). GAs have been implicated in promoting cell proliferation in growing Arabidopsis leaves (Achard et al., 2009), and reduction in the endogenous GA level can rescue the tni leaf phenotype (Fig. 8, Table 1). Mapping the tni locus shows that TNI is unlikely to encode a protein involved in GA biosynthesis or signalling (data not shown). It is more likely that the TNI product acts upstream of GA. Interestingly, a transcript encoding a GA2ox4 (AT1G02400), an enzyme that inactivates GA, is downregulated 11-fold in tni leaves in our microarray experiment, suggesting an increased GA level. However, we do not observe significant similarity between the tni transcriptome profile and the leaf transcriptome change upon GA treatment (Supplementary Table S3). It is possible that the exogenous application of GA brings about transcriptome changes different from its endogenous counterpart. The mechanism of TNI function and its link to the GA pathway will perhaps be clearer when the TNI gene is cloned and its identity is determined.
Supplementary material
Supplementary data can be found at JXB online.
Supplementary Table S1. Sequences of primers used in this study.
Supplementary Table S2. The P-values of the Student’s t-test carried out on the data shown in Table 1.
Supplementary Table S3. Comparison of transcriptome profiles.
Supplementary Figure S1. GUS expression along the medio-lateral axis.
Supplementary Figure S2. Growth kinetics of Col-0 and tni leaf length.
Supplementary Figure S3. Flattening of tni leaves by cutting.
Supplementary Figure S4. Comparison of predicted and measured P/√A values of Col-0 and tni leaves.
Supplementary Figure S5. Frequency distributions of epidermal cell size.
Supplementary Figure S6. Mutants with altered leaf size in Arabidopsis.
Supplementary Figure S7. The mitotic arrest zone in mutants with altered leaf size.
Supplementary Figure S8. Flowering time of Col-0 and tni.
Supplementary Figure S9. Surface curvature of the tni leaf is independent of leaf size.
Supplementary Figure S10. Rescue of the tni leaf phenotype by systemic application of PBZ.
Supplementary Figure S11. Global transcriptome analysis of tni leaves.
Funding
PK and KRC were supported by fellowships from the Indian Institute of Science, Bangalore; UN was supported by a grant from the Department of Biotechnology, Govt of India (BT/PR13149/BRB/10/739/2009).
Supplementary Material
Acknowledgements
We thank Prof. Imran Siddiqi for providing the plant growth facility; Jim Murray, Dirk Inzé, Detlef Weigel, Michael Lenhard, Stephen Thomas, and Pilar Cubas for gifting us the CYCD3;2::GUS, 35S::ICK2, jaw-D, klu-4, ga1-3, and tcp2-1 lines, respectively; Pooja Aggarwal and Kavitha S. Rao for help in generating the tcp2 tcp4 tcp10 mutant line; K. Ramakrishnan for help in acquiring images; J. Brahmanandam for help in making Fig. 1; and G. K. Ananthasuresh and Dibakar Sen for useful suggestions.
References
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster G T, Genschik P. 2009. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Current Biology 19, 1188–1193. [DOI] [PubMed] [Google Scholar]
- Anastasiou E, Kenz S, Gerstung M, MacLean D, Timmer J, Fleck C, Lenhard M. 2007. Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Developmental Cell 13, 843–856. [DOI] [PubMed] [Google Scholar]
- Anderson LE. 1954. Hoyer’s solution as a rapid permanent mounting medium for bryophytes. Bryologist 57, 242–244. [Google Scholar]
- Andriankaja M, Dhondt S, De Bodt S, et al. 2012. Exit from proliferation during leaf development in Arabidopsis thaliana: a not-so-gradual process. Developmental Cell 22, 64–78. [DOI] [PubMed] [Google Scholar]
- Asl LK, Dhondt S, Boudolf V, Beemster GTS, Beeckman T, Inzé D, Govaerts W, De Veylder L. 2011. Model-based analysis of Arabidopsis leaf epidermal cells reveals distinct division and expansion patterns for pavement and guard cells. Plant Physiology 156, 2172–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery. 1933. Structure and development of the tobacco leaf. American Journal of Botany, 565–592. [Google Scholar]
- Beemster GTS, Baskin TI. 1998. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiology 116, 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, De Veylder L, Vercruysse S, West G, Rombaut D, Hummelen PV, Galichet A, Gruissem W, Inzé D, Vuylsteke M. 2005. Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis . Plant Physiology 138, 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, De Vusser K, De Tavernier, De Bock K, Inzé D. 2002. Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiology 129, 854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien JC, Sussex IM. 1996. Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiology 111, 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft CE, den Boer BG, Healy JM, Murray JA. 2000. Cyclin D control of growth rate in plants. Nature 405, 575–579. [DOI] [PubMed] [Google Scholar]
- Coen E, Rolland-Lagan AG, Matthews M, Bangham JA, Prusinkiewicz P. 2004. The genetics of geometry. Proceedings of the National Academy of Sciences, USA 101, 4728–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day SJ, Lawrence PA. 2000. Measuring dimensions: the regulation of size and shape. Development 127, 2977–2987. [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, Krols L, Terras F, Landrieu I, van der Schueren E, Maes S, Naudts M, Inzé D. 2001. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. The Plant Cell 13, 1653–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, et al. 2007. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proceedings of the National Academy of Sciences, USA 104, 14537–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disch S, Anastasiou E, Sharma VK, Laux T, Fletcher JC, Lenhard M. 2006. The E3 ubiquitin ligase BIG BROTHER controls arabidopsis organ size in a dosage-dependent manner. Current Biology 16, 272–279. [DOI] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. 1999. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Developmental Biology 215, 407–419. [DOI] [PubMed] [Google Scholar]
- Efroni I, Blum E, Goldshmidt A, Eshed Y. 2008. A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. The Plant Cell 20, 2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferjani A, Horiguchi G, Yano S, Tsukaya H. 2007. Analysis of leaf development in fugu mutants of Arabidopsis reveals three compensation modes that modulate cell expansion in determinate organs. Plant Physiology. 144, 988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N, Vanhaeren H, Inzé D. 2012. Leaf size control: complex coordination of cell division and expansion. Trends in Plant Science 17, 332–340. [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Kim GT, Tsukaya H. 2005. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. The Plant Journal 43, 68–78. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T. 2008. A genetic regulatory network in the development of trichomes and root hairs. Annual Review of Plant Biology 59, 365–386. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Binkowski KA, Olszewski NE. 1996. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proceedings of the National Academy of Sciences, USA 93, 9292–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. 1993. Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. The Plant Cell 5, 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL. 2002. Arabidopsis map-based cloning in the post- genome era. Plant Physiology 129, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TR, Carpenter AE, Lamprecht MR, Moffat J, Silver S, Grenier J, Root D, Golland P, Sabatini DM. 2009. Scoring diverse cellular morphologies in image-based screens with iterative feedback and machine learning. Proceedings of the National Academy of Sciences, USA 106, 1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama T, Ichihashi Y, Murata S, Tsukaya H. 2010. The mechanism of cell cycle arrest front progression explained by a KLUH/CYP78A5-dependent mobile growth factor in developing leaves of Arabidopsis thaliana. Plant Cell Physiology 51, 1046–1054. [DOI] [PubMed] [Google Scholar]
- Kim JH, Choi D, Kende H. 2003. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. The Plant Journal 36, 94–104. [DOI] [PubMed] [Google Scholar]
- Kim Y, Schumaker KS, Zhu JK. 2006. EMS mutagenesis of Arabidopsis. Methods in Molecular Biology 323, 101–103. [DOI] [PubMed] [Google Scholar]
- Klein YK, Efrati E, Sharon E. 2007. Shaping of elastic sheets by prescription of non-euclidean metrics. Science 315, 1116–1119. [DOI] [PubMed] [Google Scholar]
- Koehl MAR, Silk WK, Liang H, Mahadevan L. 2008. How kelp produce blade shapes suited to different flow regimes: A new wrinkle. Integrative and Comparative Biology 48, 834–851. [DOI] [PubMed] [Google Scholar]
- Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M. 2010. TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. The Plant Cell 22, 3574–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyszig E. 1991. Differential geometry. New York: Dover Publications. [Google Scholar]
- Kuchen EE, Fox S, de Reuille PB, et al. 2012. Generation of leaf shape through early patterns of growth and tissue polarity. Science 335, 1092–1096. [DOI] [PubMed] [Google Scholar]
- Li J, Yang H, Peer WA, et al. 2005. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310, 121–125. [DOI] [PubMed] [Google Scholar]
- Li Y, Zheng L, Corke F, Smith C, Bevan MW. 2008. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes and Development 22, 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Mahadevan L. 2009. The shape of a long leaf. Proceedings of the National Academy of Sciences, USA 106, 22049–22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang F, Zhang H, He H, Ma L, Deng XW. 2008. Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. The Plant Journal 55, 844–856. [DOI] [PubMed] [Google Scholar]
- Masuda HP, Cabral LM, De Veylder L, Tanurdzic M, de Almeida Engler J, Geelen D, Inzé D, Martienssen RA, Ferreira PC, Hemerly AS. 2008. ABAP1 is a novel plant Armadillo BTB protein involved in DNA replication and transcription. The EMBO Journal 27, 2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Fischer RL. 2000. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proceedings of the National Academy of Sciences, USA 97, 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulia B. 2000. Leaves as shell structure: double curvature, auto-stresses, and minimal mechanical energy constrains on leaf rolling in grasses. Journal of Plant Growth Regulators 19, 19–30. [DOI] [PubMed] [Google Scholar]
- Nath U, Crawford BC, Carpenter R, Coen E. 2003. Genetic control of surface curvature. Science 299, 1404–1407. [DOI] [PubMed] [Google Scholar]
- Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Van Lijsebettens M, Kamiya Y, Inzé D, Beemster GT. 2012. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Current Biology 22, 1183–1187. [DOI] [PubMed] [Google Scholar]
- Okushima Y, Mitina I, Quach HL, Theologis A. 2005. AUXIN RESPONSE FACTOR 2 (ARF2): a pleiotropic developmental regulator. The Plant Journal 43, 29–46. [DOI] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D. 2003. Control of leaf morphogenesis by microRNAs. Nature 425, 257–263. [DOI] [PubMed] [Google Scholar]
- Powell AE, Lenhard M. 2012. Control of organ size in plants. Current Biology 22, R360–R367. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Barbier de Reuille P. 2010. Constraints of space in plant development. Journal of Experimental Botany 61, 2117–2129. [DOI] [PubMed] [Google Scholar]
- Remmler L, Rolland-Lagan AG. 2012. Computational method for quantifying growth patterns at the adaxial leaf surface in three dimensions. Plant Physiology 159, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro DM, Araújo WL, Fernie AR, Schippers JH, Mueller-Roeber B. 2012. Translatome and metabolome effects triggered by gibberellins during rosette growth in Arabidopsis . Journal of Experimental Botany 63, 2769–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RE, Mecchia MA, Debernardi JM, Schommer C, Weigel D, Palatnik JF. 2010. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 137, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvepalli K, Nath U. 2011. Hyper-activation of the TCP4 transcription factor in Arabidopsis thaliana accelerates multiple aspects of plant maturation. The Plant Journal 67, 595–607. [DOI] [PubMed] [Google Scholar]
- Schommer C, Palatnik JF, Aggarwal P, Chetelat A, Cubas P, Farmer EE, Nath U, Weigel D. 2008. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biology 6, e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schruff MC, Spielman M, Tiwari S, Adams S, Fenby N, Scott RJ. 2006. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133, 251–261. [DOI] [PubMed] [Google Scholar]
- Sessions A, Weigel D, Yanofsky MF. 1999. The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. The Plant Journal 20, 259–263. [DOI] [PubMed] [Google Scholar]
- Sharon E, Roman B, Marder M, Shin G-S, Swinney HL. 2002. Buckling cascades in free sheets. Nature 419, 579. [DOI] [PubMed] [Google Scholar]
- Silk WK. 2006. Moving with the flow: what transport laws reveal about cell division and expansion. Journal of Plant Research 119, 23–29. [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Chang C, Krol E, Sun TP. 1997. Developmental regulation of the gibberellin biosynthetic gene GA1 in Arabidopsis thaliana. The Plant Journal 12, 9–19. [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Tseng TS, Swain SM, Dill A, Jeong SY, Olszewski NE, Sun TP. 2007. Functional analysis of SPINDLY in gibberellin signaling in Arabidopsis. Plant Physiology 143, 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Straehle C, Köthe U, Hamprecht FA. 2011. ilastik: Interactive Learning and Segmentation Toolkit. 8th IEEE International Symposium on Biomedical Imaging (ISBI).
- Sun T, Goodman HM, Ausubel FM. 1992. Cloning the Aarabidopsis GA1 locus by genomic substraction.The Plant Cell 4, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PH. 1985. Gaussian curvature as a parameter of biological surface growth. Journal of Theoretical Biology 113, 63–68. [DOI] [PubMed] [Google Scholar]
- Tsuge T, Tsukaya H, Uchimiya H. 1996. Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L.) Heynh. Development 122, 1589–1600. [DOI] [PubMed] [Google Scholar]
- Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun TP. 2004. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiology 135, 1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. 2002. Arabidopsis: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- White DW. 2006. PEAPOD regulates lamina size and curvature in Arabidopsis. Proceedings of the National Academy of Sciences, USA 103, 13238–13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Heckman J, Sommerville C. 1992. Gibberellin is required for flowering in Arabidpsis thaliana under short days. Plant Physiology 100, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai O, Shani E, Russ D, Ori N. 2011. Gibberellin partly mediates LANCEOLATE activity in tomato. The Plant Journal 68, 571–582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.