Figure 2. Proposed mechanism for the direct arylation of allylic C–H bonds via photoredox and organic catalysis.
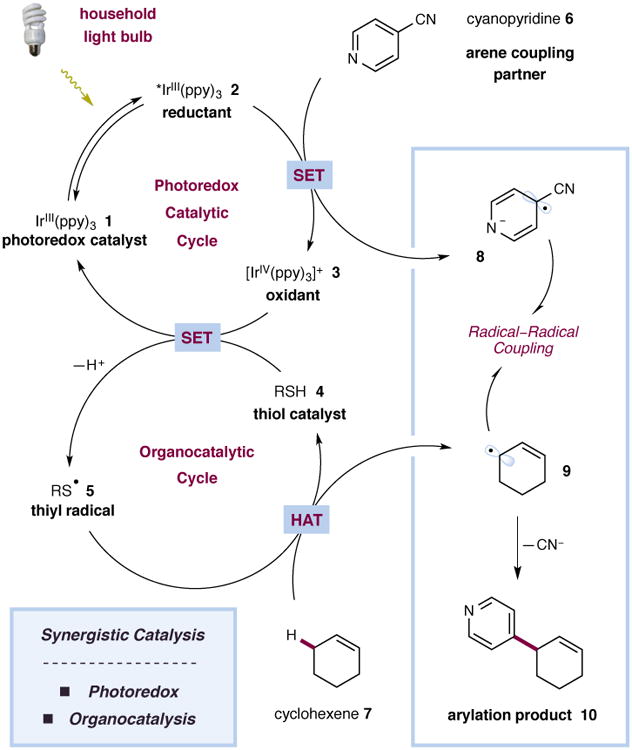
The catalytic cycle is initiated via excitation of photocatalyst 1 to give the excited state 2. Single-electron reduction of 4-cyanopyridine (6) generates the radical anion 8 along with oxidant 3. In the presence of a base, oxidant 3 is capable of oxidising the thiol catalyst 4 to give the thiyl radical 5 along with the regenerated photocatalyst 1. The thiyl radical 5 abstracts an allylic hydrogen atom from cyclohexene (7) to generate allylic radical 9. A radical–radical coupling and subsequent elimination of cyanide serve to construct the new C–C bond and form the arylation product 10.
