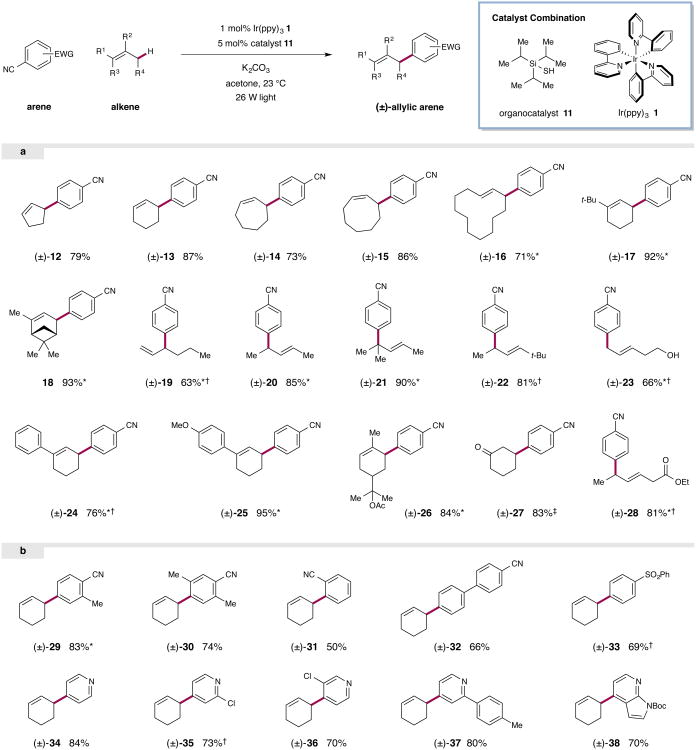
Figure 3. Substrate scope for the direct allylic arylation reaction.
A range of alkenes are efficiently arylated under the standard reaction conditions (top, generalized reaction). The substrate scope includes both cyclic and acyclic alkenes (a). A range of arenes bearing electron withdrawing substituents can be employed as coupling partners under the standard conditions (b). Isolated yields are indicated below each entry. * Isomers observed; In all cases the major isomer is depicted. Yields refer to the combined yield of all isomers. Ratios of isomers where applicable: (±)-16 (2.2:1.0 E:Z), (±)-17 (>20:1), 18 (>10:1), (±)-19 (1.4:1.0), (±)-20 (4.9:1.0), (±)-21 (1.4:1.0), (±)-23 (1.1:1.0), (±)-24 (2.1:1.0), (±)-25 (>19:1), (±)-26 (∼1:1), (±)-28 (1.2:1.0), (±)-29 (1.1:1.0). ‡ Yield from silyl enol ether (83%), yield from 2-cyclohexen-1-ol (62%). † Additional thiol or base required; See Supplementary Information for experimental details.