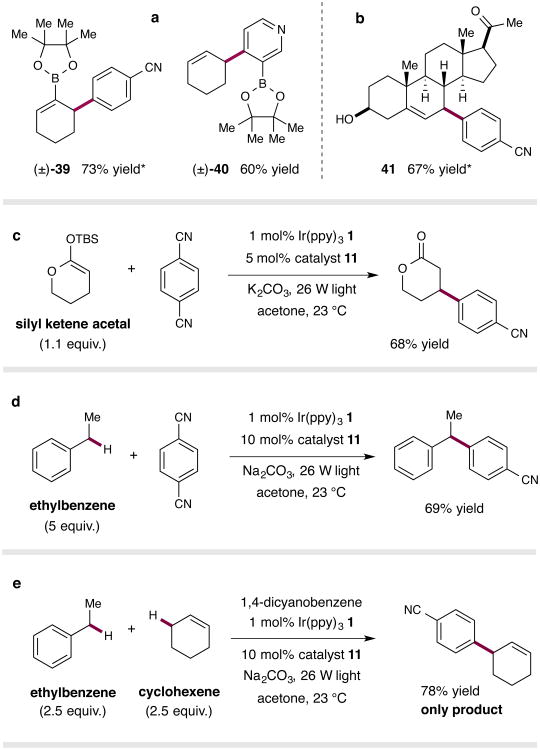
Figure 4. Expanding the scope of the direct C–H arylation protocol.
Substrates bearing boronic esters substituents are tolerated, providing a means to rapidly access functionalized building blocks (a). The mild conditions allow for late-stage functionalization of advanced synthetic intermediates and bioactive natural products (b). Silyl ketene acetals are compatible with the reaction conditions, yielding β-aryl lactones (c). Arylation is not limited to allylic C–H bonds; benzylic C–H bonds can also be arylated (d). The reactivity is governed by bond strengths, with the weaker allylic bond undergoing exclusive functionalization in a direct competition experiment (e). * Isomers observed; In all cases the major isomer is depicted. Yields refer to the combined yield of all isomers. Ratios of isomers where applicable: (±)-39 (6:1), (±)-41 (>10:1). See Supplementary Information for experimental details.