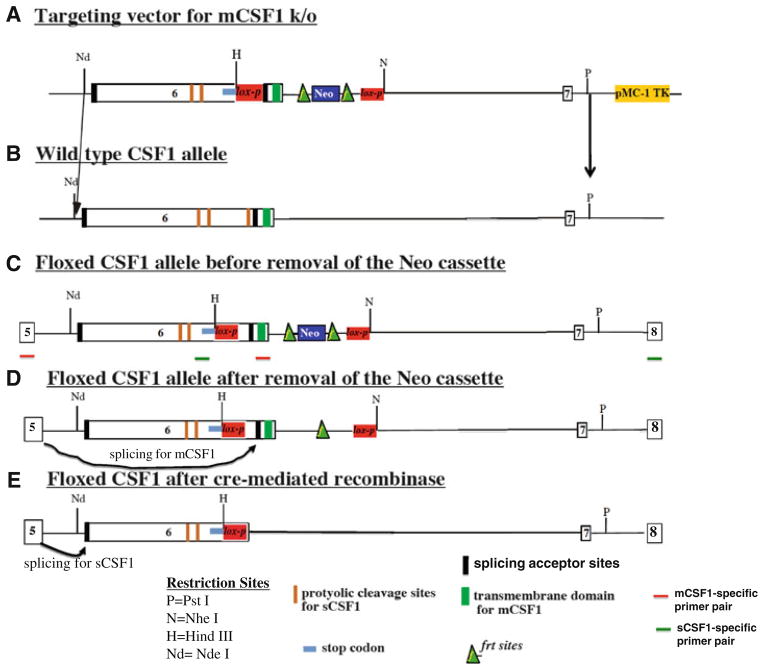
Fig. 1.
Strategy for generating conditional mCSF1 knock out mice. The targeting construct for deletion of mCSF1 introduces a stop codon followed by a lox-p site into exon 6 at a position 5′ to the splice acceptor site for mCSF1. The stop codon is designed to include all three reading frames. A Neo cassette with flanking frt sites (frt-Neo-frt-lox-p) is introduced into intron 6 (a). Excision of the Neo cassette with flp recombinase leads to a CSF1 allele that contains two lox-p sites, one in intron 6 and one, which is upstream of the splice acceptor site for mCSF1 (d). In animals bearing two of these floxed alleles (i.e. before cre-mediated recombination) both isoforms of CSF1 will be generated. Splicing to the mCSF1 acceptor site will yield a normal mCSF1 transcript (d). Splicing to the sCSF1 acceptor site will lead to generation of an mRNA that includes the sequences for the proteolyitc cleavage sites for sCSF1 followed by a stop codon. The protein product of this mRNA will yield normal mature sCSF1 since it contains the appropriate signals for proteolytic cleavage with the surrounding sequences (e). Recombination with cre leads to selective deletion of the splice acceptor site for the mCSF1 isoform as well as removal of the transmembrane domain (e). This leaves a modified exon 6 that includes the splice acceptor site for sCSF1 followed by the proteolytic sites for sCSF1, followed by a stop codon. Thus a mature functional sCSF-1 will be generated but no mCSF1 (e). b The wt allele. c The engineered allele before removal of the Neo cassette in vitro