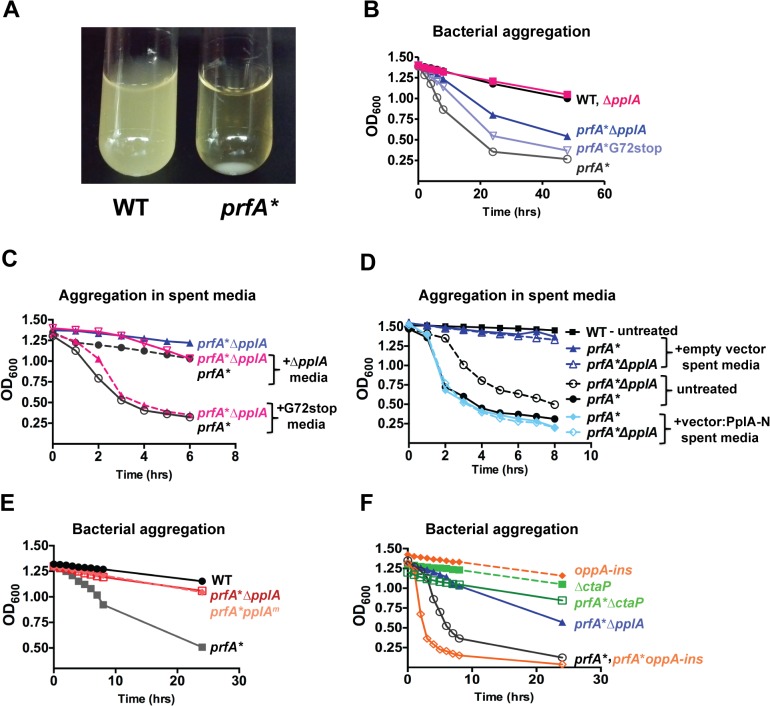
Fig 3. pPplA enhances bacterial aggregation in broth culture.
(A) Image of bacterial aggregation observed between the wild-type L. monocytogenes 10403S strain versus a prfA* mutant when bacterial cultures grown in BHI are left statically overnight at room-temperature (RT). (B) Measurement of the rate of bacterial aggregation in BHI. The optical-density at 600nm was monitored at the indicated time points for 1 mL of an overnight culture initially grown in BHI with shaking at 37°C then left statically at RT, where bacterial aggregation is measured as the decrease in the optical-density of the culture supernatant as the bacterial aggregate out of solution. (C) Measurement of bacterial aggregation of the indicated mutant strains resuspended in 1 mL of spent media derived from overnight stationary phase cultures either containing the pPplA peptide (G72stop) or lacking it (ΔpplA). The ability of the pPplA containing media (G72stop) to restore bacterial aggregation indicates the presence of a secreted substance (potentially pPplA) that enhances bacterial aggregation in broth culture. (D) Measurement of bacterial aggregation as done in panel C, except strains were resuspended in 1 mL of BHI spent media derived from E. coli containing the complementation vector construct expressing the N-terminal 72 amino acids of PplA as described in Fig. 2A or the empty vector. The ability of spent media derived from an E.coli strain containing the first 72 amino acids of pPplA supports the secretion of a PplA N-terminus derived peptide that enhances bacterial aggregation. (E) Assessment of bacterial aggregation in a strain containing three amino acid substitutions within the predicted peptide sequence (referred to as prfA*pplA m). Reduced aggregation of prfA*pplA m indicates the importance of these amino acids within the pPplA pheromone. (F) Bacterial aggregation of two oligopeptide transport mutants, a prfA* ΔctaP compared to a prfA*-oppA insertion mutant. A prfA* ΔctaP is impaired for bacterial aggregation, indicating a possible link between the pPplA peptide and import of the peptide through the CtaP transport system. For panels (B), (C) and (D), data is representative of at least three independent experiments.