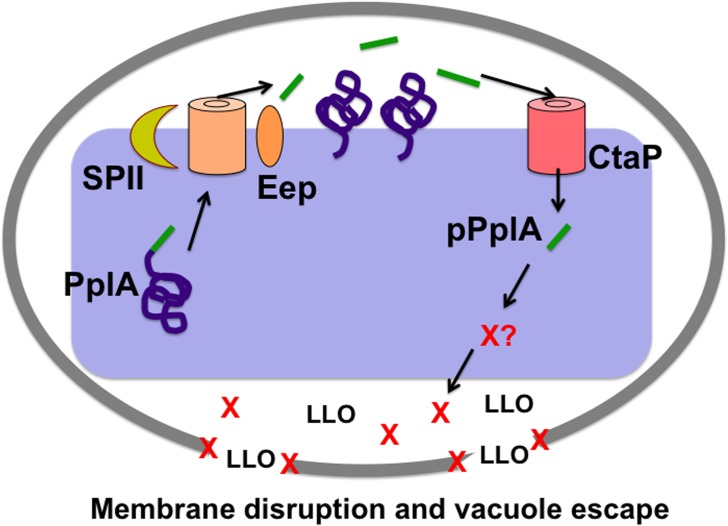
Fig 10. Model of L. monocytogenes pPplA signaling within the host vacuole.
Modeling of the predicted L. monocytogenes pPlpA signaling pathway involved in enhancing vacuole escape from the host cell vacuoles in non-professional phagocytic cells. In wild-type L. monocytogenes, pplA encodes a lipoprotein (PplA) with a peptide pheromone (pPplA) located within the N terminal secretion signal peptide (shown in green). The signal sequence of prePplA is processed by signal peptidase II (SPII) and the released signal peptide is further cleaved by the protease Eep releasing the pPplA pheromone, while the PplA protein becomes lipid modified and associated with the membrane or secreted. Upon entry of wild-type L. monocytogenes into non-professional phagocytic host cell, the confined space of the vacuole leads to import of the secreted pPplA pheromone through the CtaP peptide transporter. pPplA accumulation in the bacterial cytoplasm stimulates a signaling cascade that results in the production of an unknown factor (X) that contributes to vacuole lysis. Factor X may function by helping to stabilize the LLO generated membrane pore, facilitating eventual vacuole membrane dissolution as well as the influx of mammalian cytosol components that may promote PrfA activation and the expression of gene products required for intracellular growth and cell-to-cell spread. In the absence of pPplA (ΔpplA strains), bacterial escape is delayed until sufficient LLO and phospholipase accumulate to disrupt the vacuole membrane in the absence of factor X function. For strains containing constitutively activated PrfA*, the substantially increased secretion of LLO and the phospholipases is sufficient to disrupt the vacuole membrane in the absence of factor X.