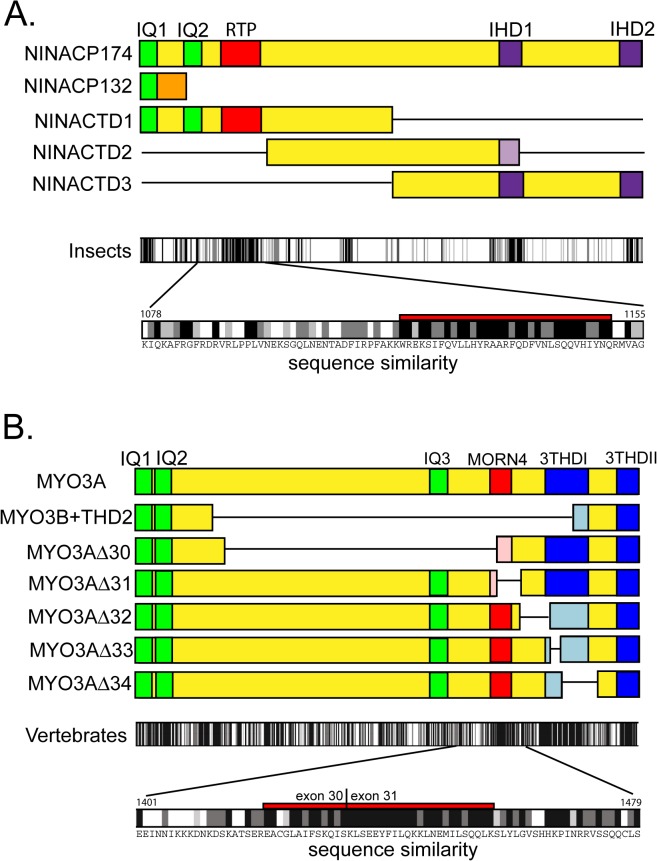
Fig 7. Identified and proposed motifs within the tail domain of Drosophila NINACp174 and human MYO3A.
(A) The top diagram shows recognized and proposed tail motifs of NINACp174: IQ1 and IQ2 (green), IHD1 and IDH2 (invertebrate homology domains, based on high sequence identity, purple), and the RTP/NINAC interaction domain (red). Displayed below this diagram are the modifications to the tail structure for NINACp132 and deletions of NINACp174 that were used to map the RTP interaction domain. The lower part of the figure summarizes amino acid sequence identities of the Drosophila NINACp174 with MYO3 proteins of other invertebrate species to highlight the level of sequence identity within the RTP interaction domain. The invertebrate species used for the analysis were Drosophila melanogaster, Drosophila yakuba, the mosquito species Aedes aegypti and Anopheles gambiae, and the honeybee Apis mellifera. Identical amino acids are shown as black bars and functionally similar amino acids as two shades of grey bars as specified by Clustalx parameters [22]. At bottom, the region containing the proposed RTP binding site is expanded to show the amino acid sequence. (B) The top diagram shows recognized and proposed tail motifs of human MYO3A: IQ1, IQ2 and IQ3 (green), 3THDI and 3THDII (tail homology domains, blue), and the MORN4/MYO3A interaction domain (red). Displayed below this diagram are the modifications to the tail structure (modified MYO3B tail and deletions of MYO3A) that were used to map of the MORN4 interaction domain. The lower part of the figure summarizes amino acid sequence identities of human MYO3A with MYO3 proteins of other vertebrate species (chimpanzee, mouse, cow, chicken) highlighting the level of sequence identity within the proposed MORN4 interaction domain. Identical and functionally similar amino acids are coded as in (A). At bottom, the region containing the proposed MORN binding site is expanded to show the amino acid sequence within the MORN4 interaction domain.