Abstract
Vestibuloplasty techniques are widely carried out to make the denture bearing area more suitable and adequate to receive the intended prosthesis. One of the major challenges after a vestibuloplasty procedure is to reduce post operative discomfort, scar contracture and subsequent loss in sulcular depth. A raw bony surface, as is obtained after Clark’s vestibuloplasty is prone not only to infections and increased pain, but also to increased scarring during the healing phase. Skin grafts have been most commonly used to cover the exposed periosteal surface but they have their own disadvantages. There is a constant search for biocompatible membranes/materials which would satisfy most criteria required of a biological scaffold. Amnion is the innermost layer of the placenta with certain unique properties. Here we discuss the efficacy of amniotic membrane as a biological dressing after vestibuloplasty.
Keywords: Amniotic membrane, Graft, Vestibuloplasty
Introduction
Vestibuloplasty is a surgical procedure whereby the oral vestibule is deepened by changing the soft tissue attachments to increase the size of the denture bearing area and height of the residual alveolar ridge [1]. Re-epithelization vestibuloplasty is indicated when the residual bone height is satisfactory but the vestibule is not ideal because of deficient or poor quality mucosa [2].
The sulcular correction should not only be adequate but should also be stable. Most conventional procedures lead to scar contracture and subsequent loss in sulcus depth. The resulting wound should heal as quickly as possible and there should be minimal discomfort during the healing period [3].
Raw surfaces overlying bone show minimal contracture. Clark’s technique aims at doing just that by leaving the raw periosteal surface to granulate instead of the labial mucosa as is the case in Kazanjian’s technique. Healing is hastened and patient discomfort is reduced by the usage of biological membranes/grafts as scaffolds [4].
Skin grafts were and are still most extensively used to cover the exposed periosteal bone surface after vestibuloplasty. A split thickness skin graft is preferred to a full thickness graft because of it better viability. However the usage of skin grafts for defects in the oral cavity has its own drawback [5]. Here we discuss the usage of amniotic membranes to cover the raw bony surface after Clark’s vestibuloplasty.
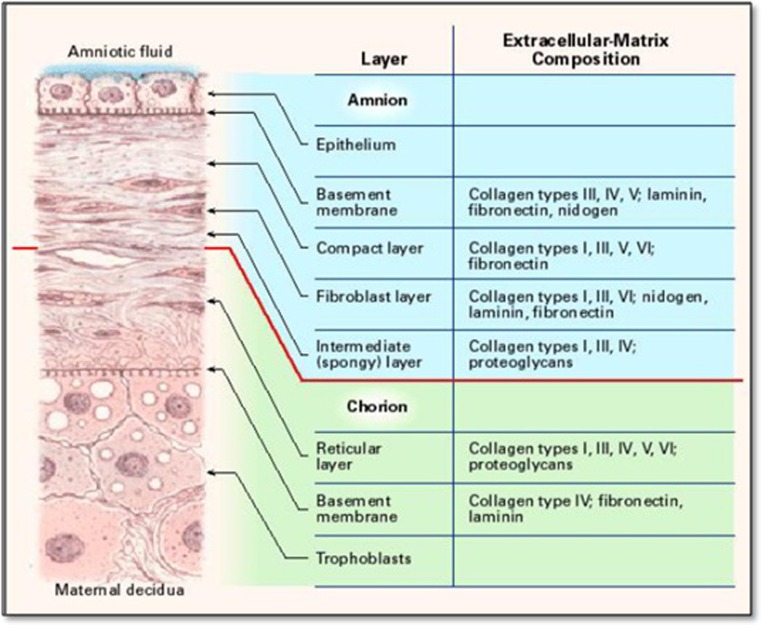
The amnion is the innermost layer of the placenta and is derived from the epiblast. Histologically it consists of a single epithelial layer, a thick basement membrane and an avascular stroma (Fig. 1). The first reported use of fetal membrane as skin substitute was by Davis in 1910 [6]. In 1913, Salbella presented the first clinical report of successful use of amniotic membrane in the treatment of burns and skin ulcerations [7, 8]. Amnion has been extensively used by ophthalmologists with a great deal of success.
Fig. 1.
Cross section view of the amniotic membrane [10]
It has been claimed to be one of the most effective biological skin substitutes used in burn wounds, with efficiency of maintaining low bacterial counts [8, 9]. It also has advantages of reducing loss of protein, electrolytes and fluids, decreasing the risk of infection, minimizing pain, acceleration of wound healing and good handling properties. Amniotic membrane has certain unique properties which make it a potentially ideal membrane/material to cover exposed periosteal bone surface after a vestibuloplasty technique. In this article we present a case in which amniotic membrane was used to cover raw periosteal surface after Clark’s vestibuloplasty procedure. We also highlight the technical aspects of harvesting, storage and usage of the amniotic membrane as a biological dressing.
Case Report
A 29 year old patient was referred from Department of Prothodontics for mandibular anterior vestibuloplasty (Fig. 2). Patient gave history of a road traffic accident which lead to avulsion of both the mandibular central incisors and the right mandibular lateral incisor. The patient was referred for correction of lower anterior sulcus depth for appropriate prosthetic rehabilitation.
Fig. 2.
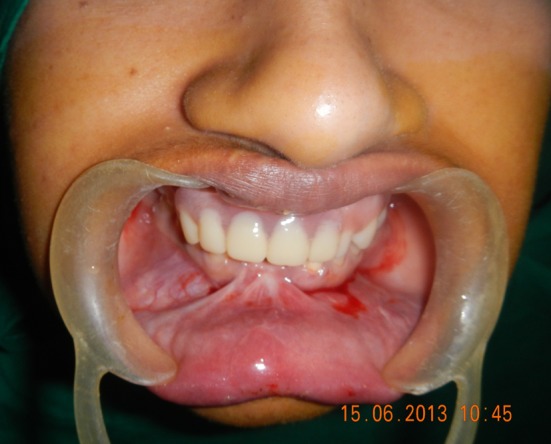
Pre-operative view
A detailed case history was recorded and it was decided to perform Clark’s vestibuloplasty to correct the sulcular depth and to use amniotic membrane to cover the raw periosteal mandibular surface. Patient was informed about the source of amniotic membrane and a written and verbal consent was taken. This study followed the Declaration of Helsinki on medical protocol and ethics. Approval was also obtained by the institutional ethical review board.
Amniotic Membrane Harvesting
Amniotic membrane is a relatively cheap and freely available tissue. An elective caesarean delivery was selected. The donor was screened and was seronegative for hepatitis B and C, syphilis and human immunodeficiency virus. Amniotic membrane was prepared a day before the procedure by separating it from chorion of placenta under sterile aseptic conditions. Processing of the amniotic membrane was carried out under sterile conditions using an antibiotic cocktail comprising 400 ml of saline containing 1,200,000 IU benzathine penicillin and 100 ml of metronidazole to cover Gram-negative bacteria, Gram-positive bacteria and fungi was used as a decontaminant and storage medium.
Preservation
The amniotic membrane was washed in a balanced salt solution containing ampicillin, streptomycin and amphotericin B. Careful delicate manual scrapping was used to clear debris off the membrane. The amniotic membrane was cleared of all blood clots and gross tissue attachment by flushing it gently with copious amounts of distilled water. The membrane was then stored in a large bottle containing 400 ml of saline containing 1,200,000 IU benzathine penicillin at 4 °C for 24 h. On the day of application it was soaked in normal saline for 10 min.
Vestibuloplasty Procedure
All the standard pre surgical protocols were followed. Using proper aseptic precautions, Clark’s vestibuloplasty was carried out under local anesthesia (2 % Lignocaine with 1:80,000 adrenaline). Care was taken during the procedure to not damage the periosteal layer. The wound was prepared as for any dressing or grafting procedure. Minimal debridement was carried out and the raw surface was irrigated with betadine and saline (Fig. 3). The area was isolated as much as possible using moist compression and total hemostasis was achieved.
Fig. 3.
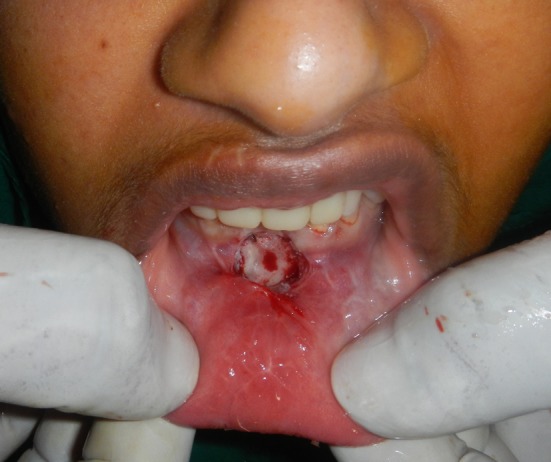
Raw bone surface after Clark’s vestibuloplasty procedure
Manipulation and Stabilization in the Oral Cavity
The amniotic membrane was manipulated so as to ensure that the mesenchymal side was placed adjacent to the raw periosteal surface. While harvesting the material from the placenta, the outer layer is the chorionic layer and the inner layer is the amniotic membrane. A small bit of the amniotic membrane can be cut and viewed under the microscope to determine the mesenchymal side of the membrane.
The membrane was gently spread and ‘teased’ across a sterile wooden block (Fig. 4). Once the appropriate size required was estimated, the membrane was placed with its mesenchymal side towards the raw periosteal surface. Care was taken to ensure proper adaptation with no intervening air bubbles. Excessive material was gently trimmed (Fig. 5). The amniotic membrane was stabilized and secured in place using 5–0 absorbable suture. A prefabricated acrylic stent was placed over the graft (Fig. 6).
Fig. 4.

Amniotic membrane spread over a wooden board
Fig. 5.
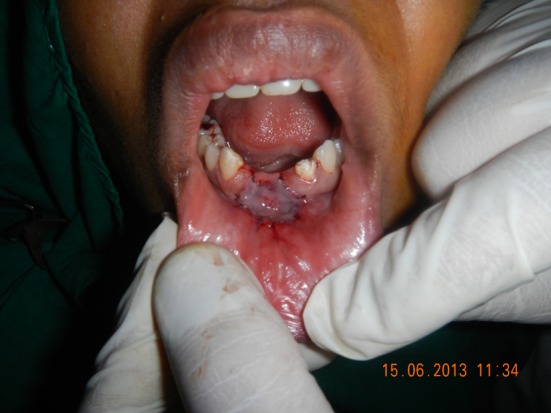
Amniotic membrane secured over the raw bone
Fig. 6.
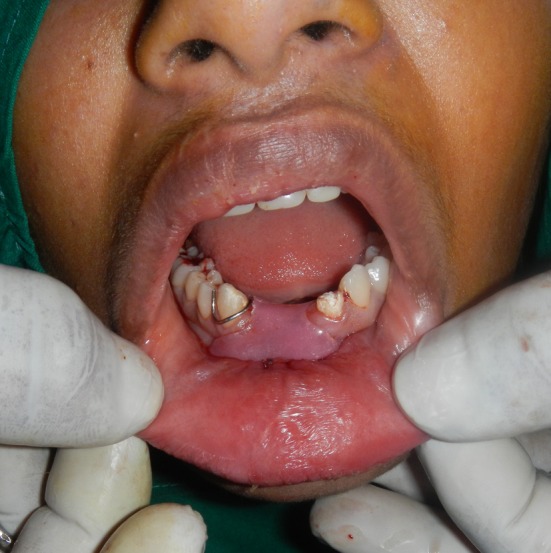
Acrylic stent secured
Patient was prescribed amoxicillin and clavulanic acid (625 mg) and metronidazole (400 mg) three times a day for 5 days.
Results
Following the removal of the splint a week after the operation, a white necrotic soft tissue layer could be seen with underlying hyperemic tissue. By the end of the second week, the necrotic layer had disappeared, leaving slightly hyperemic mucosal tissue. Three weeks after the procedure the graft area could be noticed but the amnion had completely degenerated and disappeared. The patient complained of very little discomfort and mild pain with no burning sensation. No complications such as infection or graft rejection was observed. There was minimal scarring or decrease in sulcular depth that was achieved after the vestibuloplasty procedure. After 3 weeks, the patient was referred to the department of Prosthodontics for prosthetic rehabilitation (Fig. 7).
Fig. 7.
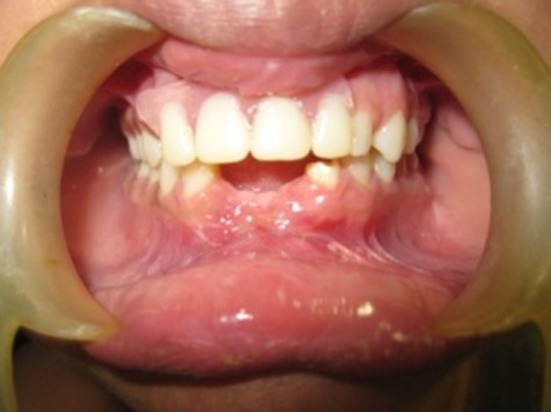
Three weeks post-operative view
Discussion
Adult wound healing is triggered by activating innate immunity and is characterized by inflammation in the acute phase, granulation tissue formation in the intermediate phase and scarring in the chronic phase. Biologic dressings, grafts are used to minimize scarring, contracture and to expedite wound healing and ensure maximum patient comfort [5]. An ideal scaffold/graft should be biocompatible. It should not be toxic, injurious or carcinogenic. It should not invoke any exaggerated immunological reaction yet should be able to invoke an appropriate host response. In addition the material should have adequate physical/mechanical properties like stability, elasticity, flexibility and appropriate resorbability at a rate congruent with tissue replacement. They should also allow for cellular adhesion and subsequent structural integrity [10].
Skin grafts have certain disadvantages. They do not ‘take’ well on denuded bone or on cortical bone. They depend on periosteal vasculature for viability. Irritation of thin immature epithelial graft causes pain, delayed healing. Skin grafts may contain hair follicles and certain patients complain of a disagreeable odour. There is a definite colour mismatch and the skin graft does not have the wetting or secretory capacity of mucosa [2, 11].
The human amnion develops from the protruding fetal ectoderm on the 8th day of a normally developing ovum [12]. Human amniotic membrane is the innermost layer of the placenta. Histologically amnion is a 0.02–0.5 mm thick, 5 layered membrane, which is equivalent to the thickness of 6–8 layers of cells, and has an average surface area of 1,600 sq cm composed of three basic layers as mentioned before [13].
Amniotic membrane has certain unique properties which makes it a viable and effective scaffold/graft material in post vestibuloplasty cases. These include [14]:
Anti inflammatory action through suppression of inflammatory mediators like cytokines, IL-1α and IL-1β and by promoting cellular apoptosis in macrophages. It also suppresses fibrosis. They evoke a minimal inflammatory response and show a lack of immunogenicity.
Antiscarring effect by its anti inflammatory action and also by suppressing TGF-β signalling at the transcription level. It also suppresses myofibroblasts proliferation and differentiation. In addition, the amniotic membrane transplant may also function as an anatomical barrier to fibrous tissue proliferation [15].
Analgesic effect: this has been observed especially when used in patients with burns. The anti-inflammatory properties are said to be the cause behind its analgesic/obtundent effect.
Antimicrobial action: the β defensins are a major group of anti microbial peptides expressed on the mucosal surfaces. Low molecular based elastase inhibitors are also expressed by the amniotic membranes which have antimicrobial properties [10].
Elective caesarean deliveries are advantageous for procurement of amniotic membranes. The placenta collected after natural vaginal delivery may have structural defects linked with stretching of the membrane during labour. They may also be infected by normal vaginal flora, herpes, chlamydia or other contaminant bacteria [16].
The cell viability of the AM depends on the media composition and storage temperature of the preservation process. Various methods have been used to preserve the AM including hypothermic storage (at 4 °C), freeze drying, sterilization, glycerol preservation and cryopreservation [10].
There is a controversy about which side of the membrane should be applied next to the periosteum. It has been observed that when the amnion’s mesenchymal side is applied to the host tissue, then vascularisation and rejection phenomenon are not seen leading to better graft survival [17].
Amniotic membrane has found useful application in burn cases. Authors have reported its usage for covering epithelial defects following flap necrosis. Guler et al. [18] concluded that grafts of amnion might be better than other grafts in mandibular vestibuloplasty because of early healing.
Conclusion
Harvesting of amniotic membrane and storage requires a lot of attention to detail and is demanding. The process is technique sensitive and manipulation of the membrane is not easy and requires experience. However rapid healing, minimal scarring, relapse and post operative pain make it a viable and desirable option. Also it is easily available and relatively cheap. It appears to be a good alternative to the other grafts and has several unique properties which make amniotic membrane an ideal biological dressing material to cover raw bone surface after vestibuloplasty procedure.
Conflict of interest
The authors would like to certify that they have no commercial associations that might pose a conflict of interest in connection with the submitted article.
References
- 1.De Koomen HA. A prosthetic view on vestibuloplasty with free mucosal grafts. Int J Oral Surg. 1977;6:38–41. doi: 10.1016/S0300-9785(77)80056-2. [DOI] [PubMed] [Google Scholar]
- 2.Starshak TJ. Preprosthetic oral and maxillofacial surgery. St Louis: Mosby; 1980. [Google Scholar]
- 3.Bruce Donoff R. Biological basis for vestibuloplasty procedures. J Oral Surg. 1976;34:890–896. [PubMed] [Google Scholar]
- 4.Huybers TJM, Stoelinga JW, Dekoomen HA. Mandibular vestibuloplasty using a free mucosal graft: a 2–7 year evaluation. Int J Oral Surg. 1985;14:11–15. doi: 10.1016/S0300-9785(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 5.Steinhauser EW. Free transplantation of oral mucosa for improvement of denture retention. J Oral Surg. 1969;27:955–961. [PubMed] [Google Scholar]
- 6.Walker AB. Use of amniotic membrane for burn wound coverage. In: Wise DL, editor. Burn wound coverings. Boca Raton: CRC Press; 1984. p. 57. [Google Scholar]
- 7.DeRoth A. Plastic repair of conjunctival defects with fetal membranes. Arch Opthalmol. 1940;23:522. doi: 10.1001/archopht.1940.00860130586006. [DOI] [Google Scholar]
- 8.Franck O, Descargues G, Menguy E. Technique of harvesting and preparation of amniotic membranes. J Fr Ophthalmol. 2000;23:729–734. [PubMed] [Google Scholar]
- 9.Mohammadi AA, Sabet B, Riazi H, Tavakkolian AR, Mohammadi MK, Iranpak S (2009) Human amniotic membrane dressing: an excellent method for outpatient management of burn wounds. IJMS 34:1, 2009.1 61
- 10.Niknejad H, Peirovi H. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 11.Tideman PJW, Stoelinga H, Huyber TJM. Mandibular vestibuloplasty using free mucosal graft. Int J Oral Surg. 1985;14:11–15. doi: 10.1016/S0300-9785(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 12.Prichard JA, Mac Donald PC, Gant NF. Williams obstetrics. 17. Englewood Cliffs: Prentice-Hall International, Inc.; 1985. p. 114. [Google Scholar]
- 13.Rao TV, Chandrasekharam V. Use of dry human and bovine amnion as a biological dressing. Arch Surg. 1981;116:891896. doi: 10.1001/archsurg.1981.01380190029007. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Sheha H. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010;5(5):645–661. doi: 10.1586/eop.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/S0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi IZ, Fareeha A, Khan WA (2010) Technique for processing and preservation of human amniotic membrane for ocular surface reconstruction. World Acad Sci Eng Technol 69:763–766
- 17.Trelford JD, Hanson FW, Anderson DO, et al. Amnion autografts, permanent structure. J Med. 1975;6:243. [PubMed] [Google Scholar]
- 18.Guler R, Ercan MT, Ulutuncel N, Devrim H, Uran N. Measurement of blood flow by the 133Xe clearance technique to grafts of amnion used in vestibuloplasty. Br J Oral Maxillofac Surg. 1997;35:280–283. doi: 10.1016/S0266-4356(97)90048-6. [DOI] [PubMed] [Google Scholar]