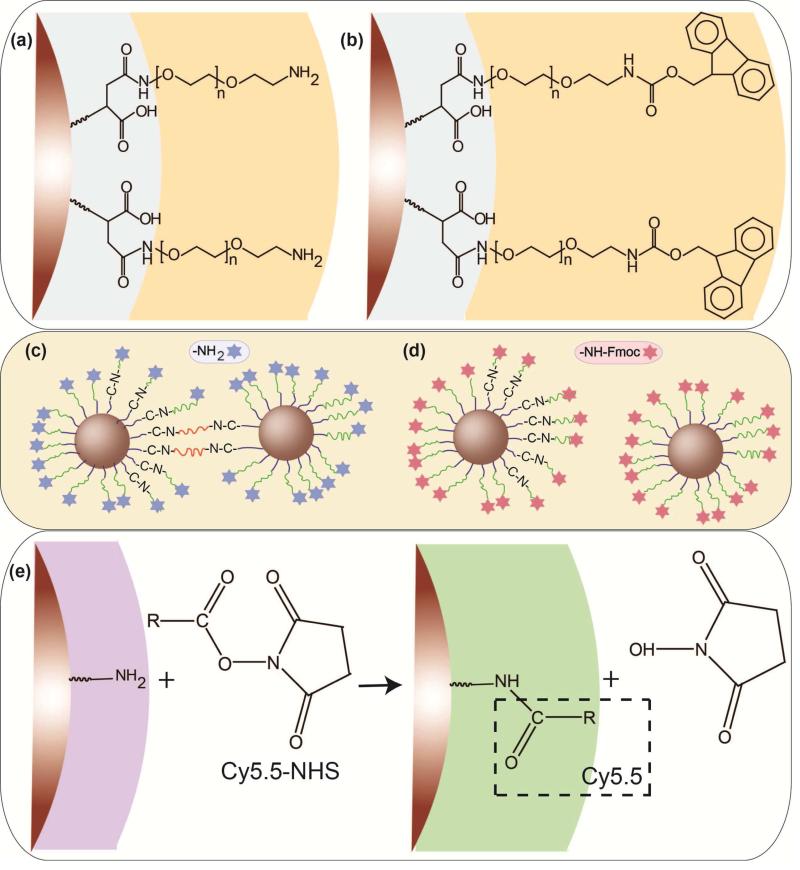
Scheme 1.
Two different surface modifications of the NPs by formation of an amide bonding between the amine groups on the PEG backbone and carboxyl groups on the surface of the silanized NPs: (a) conjugation of the bi-functional (NH2-PEG-NH2) and (b) hetero-functional (NH2-PEG-FMOC) PEG molecules. (c) Potential inter-particle bridging of some fraction of nanoparticles when bi-functional PEG is used for coating in comparison with (d) individually dispersed NPs modified with hetero-functional PEG. (e) Conjugation of amine-reactive Cy5.5 NHS ester to amine-functionalized NPs.