Abstract
Recurrence of FSGS in renal allo grafts is a major cause of graft loss. In this context, we tried to diagnose and classify FSGS in renal allografts. Indications for biopsy included graft dysfunction and/or proteinuria. Three hundred and sixty-three graft biopsies were studied over a period of 2 years. We classified FSGS into recurrent FSGS, new-onset primary FSGS and FSGS secondary to chronic humoral rejection, calcineurin inhibitor toxicity, and nephron loss and hyperfiltration injury. Twenty-four cases were diagnosed as FSGS, constituting 6.6%. Secondary FSGS was the most common FSGS in grafts in our study. Incidence of recurrent FSGS may not be accurate as pretransplant biopsy is available in very few cases.
Keywords: Focal segmental glomerulosclerosis, renal allografts, kidney transplantation
Introduction
Focal segmental glomerulosclerosis (FSGS) is a pathological term to indicate glomerular lesion associated with various etiological factors. In native kidneys, FSGS is classified as primary or secondary. In transplant kidneys, FSGS can be classified as recurrent disease or FSGS associated with chronic transplant glomerulopathy, calcineurin inhibitor (CNI) toxicity and in long-standing grafts with hyperfiltration injury. It can also occur as a new-onset primary glomerulonephritis. The incidence of recurrent FSGS is approximately 30% and secondary disease occurs in 10-20% of the cases. The distinction between these various types is difficult because of lack of pretransplant diagnosis and overlapping morphological features. We analyzed 24 graft biopsies with FSGS and tried to etiologically classify them basing on the accompanying morphological features on the graft biopsies and clinical features. It is important to diagnose the condition adequately and promptly to protect the graft and inhibit the progression of the disease.
Materials and Methods
Out of 363 allograft biopsies received between January 2012 and December 2013, 24 diagnosed as FSGS were included in the study. Indications for biopsy included graft dysfunction, increase in creatinine values, and proteinuria. Three routine stains were done in all biopsies, which included hematoxylin and eosin, periodic acid-Schiff's, and Masson's trichrome. C4d was done on all biopsies by immunohistochemical method. Immunofluorescence with immunoglobulin (Ig) G, IgM, IgA, C3c and C1q was done. Slides were reviewed by two pathologists and the diagnosis was confirmed as FSGS. Criteria to diagnose FSGS included segmental sclerosis, hyalinosis and or lipid deposition, focal or segmental collapse of the tuft with prominence of podocytes of variable degree depending on the histological type of lesion diagnosed.
Results
There were four cases of recurrent FSGS (16.6%). New-onset primary FSGS was reported in two cases (8.3%). Mean time of the presentation was 22 months after transplantation. Mean serum creatinine level was 2.7 mg/dl and mean 24 h urinary protein was 3.2 g.
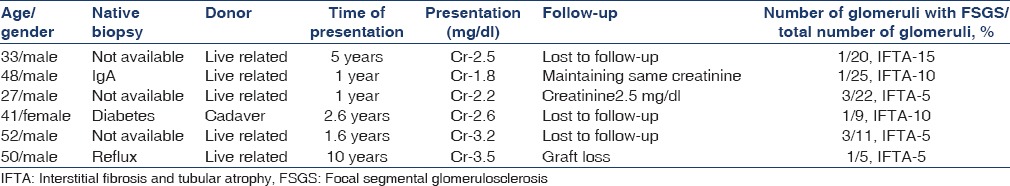
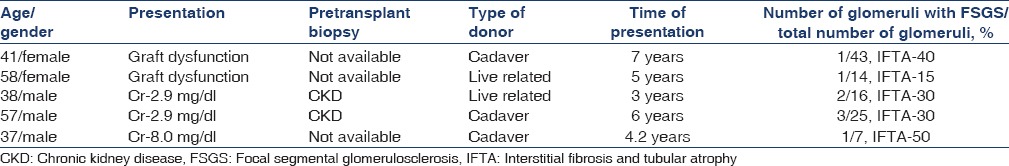
Secondary FSGS was reported in 18 cases (75%). Causes included chronic humoral rejection in seven cases (38%), CNI (cyclosporine and tacrolimus) toxicity in six cases (33%), and in long-standing grafts with hypertensive nephron loss and hyperfiltration injury in five cases (29%). All the patients were HIV-negative. Five patients were hepatitis C virus-positive. The type of donor, biochemical parameters and pretransplant diagnosis for various categories are given in Table 1.
Table 1.
Recurrent and new-onset primary diseases
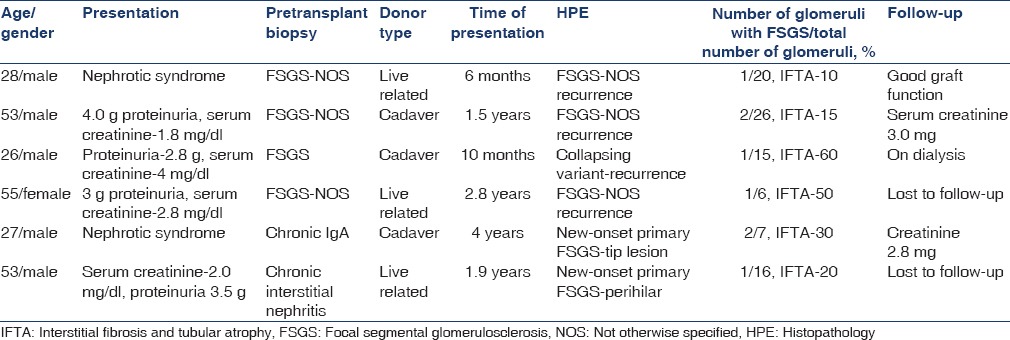
Seven cases with chronic glomerular and tubulointerstitial changes with positive C4d were classified as chronic humoral rejection. Glomerular changes included thickening and duplication of basement membrane, increase in mesangial matrix and cellularity, and focal segmental sclerosis [Figure 1]. C4d was given as positive when 10-50% of capillaries (C4d2) or more than 50% of capillaries (C4d3) showed linear circumferential staining. Clinical details are given in Table 2. Mean time of presentation was 40 months after transplantation.
Figure 1.
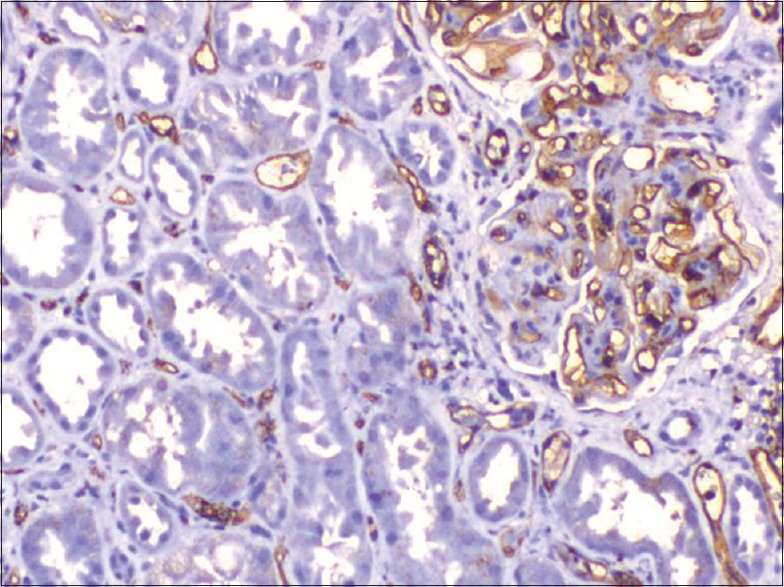
Positive C4d and segmental lesion in chronic humoral rejection (×200)
Table 2.
Chronic humoral rejection
Mean serum creatinine level was 2.7 mg/dl and 24 h urinary protein was 2.8 g. Six lesions were classified as CNI toxicity. Histological features included striped fibrosis in interstitium and nodular hyaline arteriolar sclerosis (ah2 and ah3) in vessels [Figure 2]. Glomerular changes included thickening and duplication of basement membrane. In all the cases, glomeruli showed focal and segmental sclerosis. Trough levels of the drug did not correlate with the histological findings. Other patient details are given in Table 3. Mean time of presentation was 42 months after transplantation.
Figure 2.
Nodular hyaline arteriolar sclerosis (Periodic acid-schiff, ×200)
Table 3.
Calcineurin inhibitor toxicity
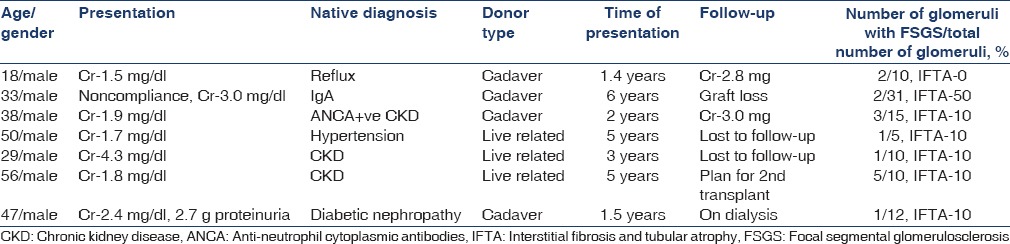
Mean serum creatinine level was 2.1 mg/dl and mean 24 h urinary protein was 800 mg. Hyperfiltration injury was diagnosed in five cases where hypertensive glomerular changes such as thickening and wrinkling of basement membrane, ischemic collapse of the tuft, and widening of Bowman's space and FSGS was seen.
Vessels showed marked subintimal fibrosis with >50% narrowing of lumen and associated with chronic tubulointerstitial changes. Mean time of presentation was 53.2 months after transplantation. Mean serum creatinine level was 2.1 mg/dl and mean 24 h urinary protein was 300 mg. Clinical and morphological details are given in Table 4.
Table 4.
Hyperfiltration injury
Discussion
Focal segmental glomerulosclerosis is a pathological term to indicate glomerular lesion associated with various clinical situations in renal allografts. FSGS is a common cause of end-stage disease and recurrence in grafts is approximately 30%.[1] We classified FSGS in renal grafts as recurrent disease, new-onset primary FSGS in cases where the native diagnosis was not FSGS, and secondary FSGS when associated with drug toxicity, chronic rejection or hypertension, depending on the clinical features and in correlation with various morphological features that are associated with FSGS. Four cases are diagnosed as recurrent disease. This number does not indicate the exact incidence, as native biopsy diagnosis is not available in many cases.
Risk of recurrence includes young age of the patient, presence of mesangial proliferation, and rapid progression to end-stage disease and pretransplant bilateral nephrectomy.[2] Recurrence occurs in renal grafts in early post-transplant period. These patients present with heavy proteinuria usually of nephrotic range. This criterion and an available diagnosis of native disease are helpful in diagnosing recurrent disease. In our series, all the four cases had a biopsy diagnosis of FSGS. Recurrence occurred in the 1st year of transplantation. The diagnosis of recurrent disease requires accurate classification. Recurrent disease in 81% of cases has similar morphology to native disease.[3] In our study, the type of FSGS in native kidney was available in three cases, and recurrence disease had a similar pattern to native disease. One patient had collapsing glomerulopathy [Figure 3]. Recurrence can be classified as recurrence of same variant (51%), recurrence of same variant preceded by minimal change disease (19%) and recurrence with a different variant of FSGS (19%).[4] Graft survival is poor and progression to end-stage renal disease is rapid and is as high as 50%.[5] It is therefore important to diagnose and differentiate this entity from other causes of FSGS.
Figure 3.
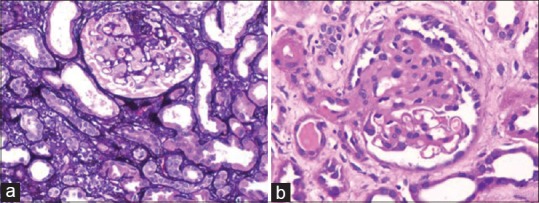
(a) Collapsing glomerulopathy (Jones, ×200). (b) Focal segmental glomerulosclerosis - not otherwise specified (H and E, ×200)
Chronic transplant glomerulopathy and chronic humoral rejection are other conditions in which FSGS can occur. It is the most common cause in our series. The mechanism for this is chronic immunological glomerular endothelial and podocyte injury, which is resulting in FSGS.[6] This particular lesion is relatively easy to diagnose as associated features of chronic graft damage are seen, and C4d is positive. These patients presented in late post-transplant period mostly after 3 years and presentation is due to the rise in creatinine and mild proteinuria.
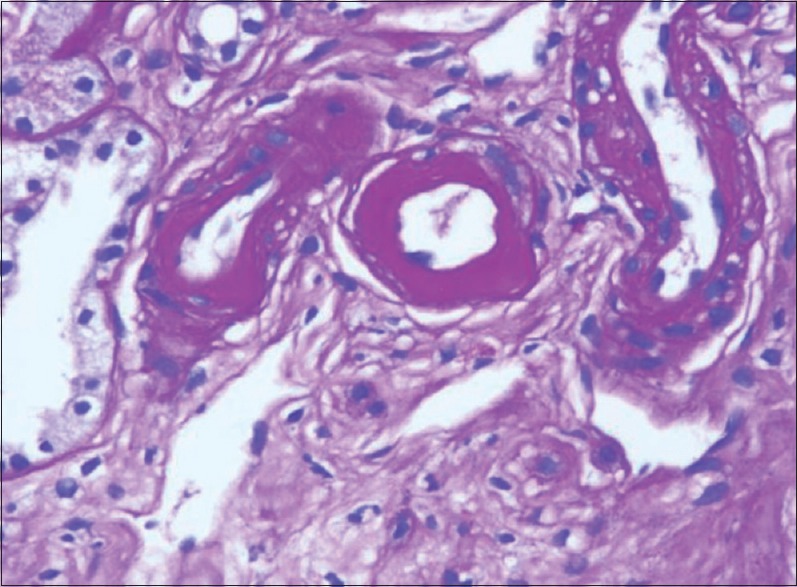
Chronic CNI toxicity is another condition that causes morphological abnormality, FSGS. These lesions are purely based on pathology diagnosis as serum levels do not correlate with the extent of renal damage[7] and clinical features are not specific. Glomerular changes include glomerular hypertrophy, capillary collapse, focal segmental or focal global sclerosis. FSGS results from hyperfiltration injury due to loss of functioning nephrons rather than direct toxic effects on podocytes.[7] Presence of hyaline arteriopathy, stripped fibrosis, [Figure 4] tubular atrophy with or without isometric vacuolation, and absence of heavy proteinuria help to diagnose this particular entity.
Figure 4.
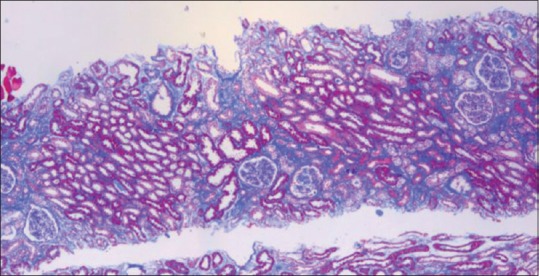
Striped fibrosis (Masson's trichrome, ×100)
The true incidence of glomerulonephritis in grafts is not exactly known and ranges from 4% to 20%.[8] Membranous nephropathy and FSGS are most common of the glomerulonephropathies that can be seen in grafts with an incidence of 1-9%.[9] We diagnosed two cases of new-onset FSGS in patients presenting with nephrotic range proteinuria. The native disease in these patients was not FSGS. Histologically, there were no features of hypertension or CNI toxicity and C4d was negative. New-onset FSGS has favorable clinical course[10] when compared with recurrent disease and hence it is important to identify this lesion.
In long-standing grafts with nephron loss and chronic tubulointerstitial changes, FSGS occurs as a result of hypertensive vascular changes and hyperfiltration injury. Nephron loss and chronic tubulointerstitial changes are seen in drug toxicity in allografts. We described this entity separately when arterial changes are predominant and tell-tale signs of CNI toxicity are absent.
Conclusions
Focal segmental glomerulosclerosis is a morphological expression of various conditions in renal allografts. An attempt was made to etiologically classify these lesions as there is a difference in treatment and prognosis. Secondary FSGS was the most common in our series. It is important to make a diagnosis carefully using clinical information and pathology when a decision about second transplant has to be made.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5:2363–72. doi: 10.2215/CJN.06720810. [DOI] [PubMed] [Google Scholar]
- 2.Pardon A, Audard V, Caillard S, Moulin B, Desvaux D, Bentaarit B, et al. Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrol Dial Transplant. 2006;21:1053–9. doi: 10.1093/ndt/gfk005. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu A, Higo S, Fujita E, Mii A, Kaneko T. Focal segmental glomerulosclerosis after renal transplantation. Clin Transplant. 2011;25(Suppl 23):6–14. doi: 10.1111/j.1399-0012.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- 4.IJpelaar DH, Farris AB, Goemaere N, Amann K, Goldschmeding R, Nguyen TQ, et al. Fidelity and evolution of recurrent FSGS in renal allografts. J Am Soc Nephrol. 2008;19:2219–24. doi: 10.1681/ASN.2007121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artero M, Biava C, Amend W, Tomlanovich S, Vincenti F. Recurrent focal glomerulosclerosis: Natural history and response to therapy. Am J Med. 1992;92:375–83. doi: 10.1016/0002-9343(92)90267-f. [DOI] [PubMed] [Google Scholar]
- 6.Cosio FG, Frankel WL, Pelletier RP, Pesavento TE, Henry ML, Ferguson RM. Focal segmental glomerulosclerosis in renal allografts with chronic nephropathy: Implications for graft survival. Am J Kidney Dis. 1999;34:731–8. doi: 10.1016/S0272-6386(99)70400-2. [DOI] [PubMed] [Google Scholar]
- 7.Liptak P, Ivanyi B. Primer: Histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol. 2006;2:398–404. doi: 10.1038/ncpneph0225. [DOI] [PubMed] [Google Scholar]
- 8.Kotanko P, Pusey CD, Levy JB. Recurrent glomerulonephritis following renal transplantation. Transplantation. 1997;63:1045–52. doi: 10.1097/00007890-199704270-00001. [DOI] [PubMed] [Google Scholar]
- 9.Woolley AC, Rosenberg ME, Burke BA, Nath KA. De novo focal glomerulosclerosis after kidney transplantation. Am J Med. 1988;84:310–4. doi: 10.1016/0002-9343(88)90431-7. [DOI] [PubMed] [Google Scholar]
- 10.Sprangers B, Kuypers DR. Recurrence of glomerulonephritis after renal transplantation. Transplant Rev (Orlando) 2013;27:126–34. doi: 10.1016/j.trre.2013.07.004. [DOI] [PubMed] [Google Scholar]


