Abstract
Introduction:
The purpose of this study was to compare solubility of a new calcium silicate-based cement, Biodentine with three commonly used root-end filling materials viz. glass-ionomer cement (GIC), intermediate restorative material (IRM), and mineral trioxide aggregate (MTA).
Materials and Methods:
Twenty stainless steel ring molds were filled with cements corresponding to four groups (n = 5). The weight of 20 dried glass bottles was recorded. Samples were transferred to bottles containing 5 ml of distilled water and stored for 24 h. The bottles were dried at 105C and weighed. This procedure was repeated for 3, 10, 30, and 60 days. Data was analyzed with one-way analysis of variance (ANOVA) test (P < 0.05).
Results:
Biodentine demonstrated significantly higher solubility than MTA for 30- and 60-day immersion periods. Statistical difference was noted between the solubility values of Biodentine samples amongst each of the five time intervals.
Conclusions:
Biodentine exhibited higher solubility in comparison with all other cements.
Keywords: Calcium chloride, calcium silicate, glass-ionomer cement, periapical, root-end filling materials, root-end filling, solubility
INTRODUCTION
The goal of any root-end filling material has been firmly established in scientific literature. It should create a seal, thus avoiding bacterial infiltration and diffusion of bacterial toxins from the root canal system to periradicular tissues.[1] Amongst other desirable properties it should also be dimensionally stable, nonabsorbable, not affected by the presence of moisture, and insoluble.[2,3] Lack of solubility has been mentioned as one of the ideal characteristics of root-end filling material.[4] ISO 6876 standard places the acceptable limit of weight loss for solubility test at 3%.[5] A myriad of dental materials have been extensively investigated for solubility if used as a root-end filling material. Studies done by Poggio et al.,[6] Torabinejad et al.,[7] and Danesh et al.,[8] have established low or no solubility of mineral trioxide aggregate (MTA).
Torabinejad et al.,[7] observed slight solubility of intermediate restorative material (IRM) in their investigation and were of the opinion that it was clinically insignificant. Pieper et al.,[9] have reported high values of solubility, whereas Poggio et al.,[6] have observed low solubility of IRM in water upto 2 months and have termed the cement as virtually insoluble. Glass-ionomer cements (GIC) have still been retained as viable alternatives to MTA because of their ability to bond with dentin, biocompatibility, antimicrobial activity, and favorable cost-benefit analysis.[10] Pieper et al.,[9] have reported very low solubility values of GIC as compared to IRM.
Amongst the available root-end filling materials, MTA has gained popularity as it has not only shown low solubility but also a superior sealing ability than IRM,[11] with a favorable periapical tissue response than IRM.[12] However, MTA has exhibited specific disadvantages of long setting time and difficult handling characteristics. The long setting time may favor solubility and/or disintegration or displacement from the retrograde cavity.[13] Lately, Biodentine (Septodont, France); another material based on the calcium-silicate formulation has been introduced which is intended as an alternative to MTA with specific advantages such as a short setting time, high mechanical strength, and superior handling characteristics. Its applications are the usual indications of this class of calcium-silicate cements such as MTA viz. endodontic repair of perforations and resorptions, apexification, root-end fillings, and vital pulp therapy.
Although initial scientific reports have demonstrated favorable results, the use of Biodentine as a root-end filling material remains uninvestigated. No studies have reported solubility of Biodentine over a period of 60 days. The aim of the present study, therefore, was to compare solubility of a new calcium silicate-based cement with three commonly used root-end filling materials viz. GIC, IRM, and MTA over 1, 3, 10, 30, and 60 days. The null hypothesis was that none of the materials tested were soluble.
MATERIALS AND METHODS
Solubility was determined in accordance with the International Standards Organization (ISO) 6876 method and with the American Dental Association (ADA) specification # 30.[5,14] The methods used in the current investigation. Twenty specially fabricated stainless steel ring molds with an internal diameter of 20 ± 0.1 mm and a height of 1.5 ± 0.1 mm were used for sample preparation. All the molds were cleaned with acetone in an ultrasound bath for 15 min. They were then left to dry for 30 min. The ring molds were filled with test materials/cements corresponding to four groups (n = 5) viz. GC Fuji IX (GC, Tokyo, Japan), IRM (Caulk, Dentsply, Milford, DE), Pro Root MTA (Maillefer, Dentsply, Switzerland), and Biodentine (Septodont, France). The restorative materials were mixed by the same operator in accordance with manufacturers’ instructions. The ring molds were placed on a glass slab and filled to excess with the mixed materials. Another glass plate covered with a Mylar strip was lightly placed on top of the molds to remove any excess material. Samples were left to set in a humidor and placed inside an incubator cabinet maintained at 37°C. At the end of 24 h, samples were removed from the cabinet and exposed to air for 15 min. Each sample was then weighed three times to record the average reading. This weight was noted as initial dry weight (IDW) of the specimens. A GP 600 P Sartorius balance (accuracy, 0.0001 g) was used for all measurements.
Individual weight of 20 dried and labeled amber colored glass bottles was recorded as dry bottle weight (DBW). Samples were then transferred to these individual bottles containing 5 ml of distilled water. The bottles were stored in an incubator maintained at 37°C for a period of 24 h. After removal of bottles from the incubator, each sample was rinsed with 15 ml of distilled water with the help of a syringe. During the rinsing process the samples were not removed from the bottles. Excess water remaining in the bottles was evaporated in an oven by maintaining a temperature slightly below boiling point. The bottles were then dried in the oven at 105°C and cooled down in the same desiccators. Each bottle was then individually weighed and recorded as final dry weight (FDW). Solubility of each sample was calculated by the following formula and was expressed as a percentage value of IDW.[6]
Solubility = FDW −DBW × 100
After recording the FDW at 24 h period, distilled water was added to the bottles and they were transferred to the incubator until they were evaluated at intervals of 3, 10, 30, and 60 days by using the same method. The data was subjected to the statistical analysis using Statistical Package for Social Sciences (SPSS) software, v.11.0 (SPSS Inc, Chicago, IL) and subjected to one-way analysis of variance (ANOVA) test with the statistical difference set at P < 0.05.
RESULTS
The results of solubility test [Figure 1] with statistical comparison of mean and standard deviation between the groups at different time intervals are listed in [Tables 1-5]. Statistical difference was noted between the solubility values of Biodentine samples amongst each of the five time intervals. Solubility of Biodentine in comparison with all other cements upto 10-day exposure times showed no statistical difference. Biodentine showed acceptable solubility values at exposure times of 24 h, 3 days, and 10 days. Thereafter it demonstrated a marked increase in solubility; 1.07 (day 10 versus day 30) and 2.89 (day 30 versus day 60). At exposure times of upto 30 days (1, 3, 10, and 30 days), IRM showed least solubility values amongst all test cements. GIC showed significantly higher solubility than MTA for 10 day immersion period. Solubility values of GIC at all other exposure times were statistically nonsignificant in comparison to other cements.
Figure 1.
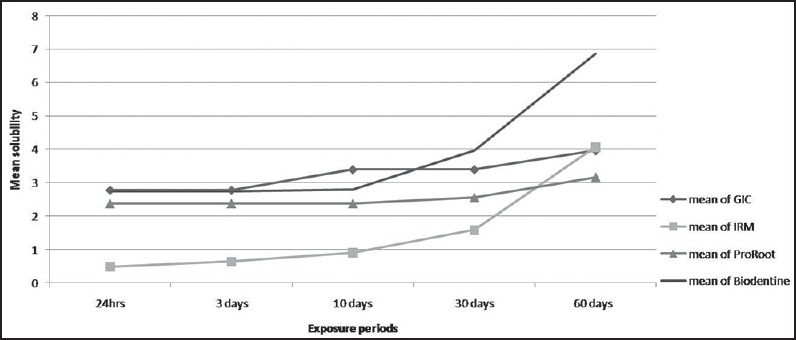
Graph depicting mean solubility of the root-end filling materials at various exposure periods. GIC = Glass-ionomer cement, IRM = Intermediate restorative material
Table 1.
Comparison of mean and standard deviations of the solubility for different test cements at 24 h period

Table 5.
Comparison of mean and standard deviations of the solubility for different test cements at 60 day period

Table 2.
Comparison of mean and standard deviations of the solubility for different test cements at 3 day period

Table 3.
Comparison of mean and standard deviations of the solubility for different test cements at 10 day period

Table 4.
Comparison of mean and standard deviations of the solubility for different test cements at 30 day period

DISCUSSION
Root-end filling materials are usually in contact with periradicular tissue fluid until they are eventually covered by fibrous connective tissue or cementoid.[7] However, it is important to determine solubility of test groups for longer periods, as some materials might be stable immediately after setting, but suffer disintegration with time and longer time span indicates more certainty of results.[15] Longer immersion period was used according to studies of Fridland and Rosado,[16] allowing observation of solubility behavior for longer period of time.
Solubility was tested for upto 60 days in the present study as the periapical tissue goes through a dynamic healing phase immediately following periapical surgery. An osseous excisional wound healing study in monkeys at postsurgical interval of 28 days demonstrated continual replacement of woven bone by maturing new trabecular bone.[17] A study by Cardaropoli G et al.,[18] in dogs showed that events such as formation of coagulum, inflammatory cell migration, and granulation tissue formation occurred over the first 7 days; provisional connective tissue formation and woven bone formation occurred at 14 and 30 days, respectively; and modeling and remodeling of this woven bone into lamellar bone was seen at 60 day interval. It can be theorized that leaching of components from the root-canal filling during this period may have biologic effects on the surrounding tissues,[19,20] especially during the immediate postsurgical healing phase. Moreover, solubility may be a cause of disintegration or degradation of the root-end filling material, thereby leaving spaces that may provide gaps for bacterial colonization and their passage in periapical tissues.[21]
Principal components of the Biodentine powder are tricalcium silicate, calcium carbonate and zirconium oxide for radiopacity. The liquid consists of calcium chloride as the setting accelerator and water reducing agent.[22,23] According to the manufacturers’ claims, Biodentine differs from other cements in its class by higher purity of calcium silicate and absence of any aluminate in the final product, which results in superior mechanical properties.
The mixing procedure and pressure used for compaction and environment humidity is not easy to control and reproduce. Some of these factors are addressed in Biodentine due to pre-proportioned capsules, single dose containers, and automatic mixing. However, some degree of porosity is characteristic of dental cements prepared by mixing the powder and liquid due to incorporation of microscopic air bubbles during the mixing operation.[24] Porosities resulting from this may hold moisture, thus resulting in solubilization of the components.[25]
Addition of calcium chloride (CaCl2) to calcium silicate-based cements is used for reducing the setting time. A study by Bortoluzzi et al.,[25] has proven that addition of 10% CaCl2 reduces the setting time and solubility of white MTA without promoting its disintegration. The investigators attributed this reduced solubility to penetration of CaCl2 in the pores of the cement leading to accelerated hydration, and thus a faster crystallization of the cement. Considering the similarities between ProRoot MTA and Biodentine, one would expect similar results from Biodentine.
However, the results of our study were not in accordance with Bortoluzzi et al.,[25] Although statistically insignificant, Biodentine and MTA showed comparable solubility values for evaluation periods of upto 10 days. Significantly higher solubility of Biodentine as compared to MTA was observed for 30 and 60 day immersion periods. It can be theorized that for a material releasing calcium ions to exert a biologic effect, it must, to some extent solubilize and dissociate from fully hardened cement, thus resulting in disintegration.[3] In fact, a study done by Han et al,[22] to compare Ca and Si uptake by elemental mapping and line-scan analysis of root canal dentin adjacent to ProRoot and Biodentine in the presence of phosphate-buffered saline revealed; wider Ca and Si-rich dentin areas and larger incorporation depths with Biodentine samples than MTA. They attributed their findings to higher amounts of Ca and Si dissolution in Biodentine than in MTA. The greater solubility of Biodentine in our study could be explained due to possibly higher dissolution of ions than MTA.
It is interesting to know that Biodentine shows biomimetic remineralization and causes deposition of calcium phosphate on the surface. This suggests a high rate of calcium release with fast formation of apatite and makes Biodentine a scaffold for clinical healing.[26] Attik et al.,[27] in an in vitro biocompatibility study on osteoblast-like cells have shown that cell attachment and cell proliferation on Biodentine is comparable to MTA. Additionally Kim et al.,[28] have shown that Biodentine causes deposition of amorphous calcium phosphate interfacial layer with radicular dentin and that the Ca/P ratio of this layer is comparable to MTA.
As for IRM, our results are in agreement with the studies of Poggio et al.,[6] IRM showed least solubility amongst all filling materials upto 30 days. IRM showed significantly higher solubility at 60 day period compared to 24 h period. These results could be attributed to previously reported findings by Pieper et al.,[9] who have shown higher solubility that this cement undergoes when in prolonged contact with moisture. This process has been explained by Wilson and Batchelor,[29] as eugenol loss of cement matrix by aqueous leaching resulting in microstructural degradation.
Although the methodology for ascertaining solubility closely mimics clinical situation, yet the results can only be partly transferred to a clinical situation. Only a part of the cements are exposed to the periapical fluids as against the study conditions where the surface area exposed to the aqueous environment is much greater.[6] All the materials were tested for solubility after they completely set; therefore, these test conditions differ from any clinical situation where the materials are used before their initial setting. As pointed out by Kaplan et al.,[15] sealers when used in endodontic therapy come in contact with periapical fluids immediately; however, they are not completely immersed in it. A similar clinical scenario can be correlated to the use of root-end filling materials.
CONCLUSION
When compared to MTA, though Biodentine solubility values were higher this solubility occurs only at the surface which is exposed to the solution and causes negligible dimensional change. With recent reports consistently suggesting bioimmetic remineralization and deposition of interfacial layer with the root dentin, solubility of Biodentine may be working in favor of the material than against it. Future studies of Biodentine should aim at analyzing the association between solubility with remineralization and stimulation of cells to better understand whether solubility is one of the contributing factors towards clinical healing. In effect the null hypothesis was rejected and it was concluded that Biodentine exhibited higher solubility in comparison with other test cements.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Costa AT, Konrath F, Dedavid B, Weber JB, de Oliveira MG. Marginal adaptation of root- endfilling materials: An in vitro study with teeth and replicas. J Contemp Dent Pract. 2009;10:75–82. [PubMed] [Google Scholar]
- 2.Aqrabawi J. Sealing ability of amalgam, super EBA cement, and MTA when used as 1. retrograde filling materials. Br Dent J. 2000;188:266–8. doi: 10.1038/sj.bdj.4800450. [DOI] [PubMed] [Google Scholar]
- 3.Vivan RR, Zapata RO, Zeferino MA, Bramante CM, Bernardineli N, Garcia RB, et al. Evaluation of the physical and chemical properties of two commercial and three experimental root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:250–6. doi: 10.1016/j.tripleo.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Grossman LI. Solubility of root canal cements. J Dent Res. 1978;57:927. doi: 10.1177/00220345780570092001. [DOI] [PubMed] [Google Scholar]
- 5.Geneva: International Organization for Standardization; 2001. International Organization for Standardization. Specification for dental root canal sealing materials: ISO 6876. [Google Scholar]
- 6.Poggio C, Lombardini M, Alessandro C, Simonetta R. Solubility of root-end-filling materials: A comparative study. J Endod. 2007;33:1094–7. doi: 10.1016/j.joen.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 8.Danesh G, Dammaschke T, Gerth HU, Zandbiglari T, Schäfer E. A comparative study of selected properties of ProRoot mineral trioxide aggregate and two Portland cements. Int Endod J. 2006;39:213–9. doi: 10.1111/j.1365-2591.2006.01076.x. [DOI] [PubMed] [Google Scholar]
- 9.Pieper CM, Zanchi CH, Rodrigues-Junior SA, Moraes RR, Pontes LS, Bueno M. Sealing ability, water sorption, solubility and tooth brushing abrasion resistance of temporary filling materials. Int Endod J. 2009;42:893–9. doi: 10.1111/j.1365-2591.2009.01590.x. [DOI] [PubMed] [Google Scholar]
- 10.De Bruyne MA, De Bruyne RJ, Rosiers L, De Moor RJ. Longitudinal study on microleakage of three root-end filling materials by the fluid transport method and by capillary flow porometry. Int Endod J. 2005;38:129–36. doi: 10.1111/j.1365-2591.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- 11.Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root end filling materials: Effects of blood contamination. J Endod. 1994;20:159–63. doi: 10.1016/S0099-2399(06)80326-2. [DOI] [PubMed] [Google Scholar]
- 12.Wälivaara DÅ, Abrahamsson P, Isaksson S, Salata LA, Sennerby L, Dahlin C. Periapical tissue response after use of intermediate restorative material, gutta-percha, reinforced zinc oxide cement, and mineral trioxide aggregate as retrograde root-end filling materials: A histologic study in dogs. J Oral Maxillofac Surg. 2012;70:2041–7. doi: 10.1016/j.joms.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological 1. environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25:787–93. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 14.ANSI/ADA. Revised American National Standard/American Dental Association specification no. 30 for dental zinc oxide eugenol and zinc oxide non-eugenol cements 7.3. 2001 [Google Scholar]
- 15.Kaplan AE, Goldberg F, Artaza LP, de Silvio A, Macchi RL. Disintegration of endodontic cements in water. J Endod. 1997;23:439–41. doi: 10.1016/S0099-2399(97)80298-1. [DOI] [PubMed] [Google Scholar]
- 16.Fridland M, Rosado R. MTA solubility: A long term study. J Endod. 2005;31:376–9. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 17.Harrison JW, Jurosky KA. Wound healing in the tissues of the periodontium following periradicular surgery. III. The osseous excisional wound. J Endod. 1992;18:76–81. doi: 10.1016/S0099-2399(06)81375-0. [DOI] [PubMed] [Google Scholar]
- 18.Cardaropoli G, Araûjo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003;30:809–18. doi: 10.1034/j.1600-051x.2003.00366.x. [DOI] [PubMed] [Google Scholar]
- 19.Orstavik D. Weight loss of endodontic sealers, cements and pastes in water. Scand J Dent Res. 1983;91:316–9. [PubMed] [Google Scholar]
- 20.Geurtsen W, Leyhausen G. Biological aspects of root canal filling materials — Histocompatibility, cytotoxicity, and mutagenicity. Clin Oral Investig. 1997;1:5–11. doi: 10.1007/s007840050002. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TN. Obturation of the root canal system. In: Cohen S, Burns RC, editors. Pathways of the Pulp. 6th ed. St. Louis: Mosby; 1994. pp. 219–71. [Google Scholar]
- 22.Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J. 2011;44:1081–7. doi: 10.1111/j.1365-2591.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 23.Laurent P, Camps J, De Méo M, Déjou J, About I. Induction of specific cell responses to a Ca (3) SiO (5)-based posterior restorative material. Dent Mater. 2008;24:1486–94. doi: 10.1016/j.dental.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with 1. different water-to-powder ratios. J Endod. 2003;29:814–7. doi: 10.1097/00004770-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009;35:550–4. doi: 10.1016/j.joen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Gandolfi MG, Siboni F, Botero T, Bossù M, Riccitiello F, Prati C. Calcium silicate and 1. calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater. 2014 doi: 10.5301/jabfm.5000201. [DOI] [PubMed] [Google Scholar]
- 27.Attik GN, Villat C, Hallay F, Pradelle-Plasse N, Bonnet H, Moreau K, et al. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblasts cells: Biodentine™ versus MTA. Int Endod J. 2014;47:1133–41. doi: 10.1111/iej.12261. [DOI] [PubMed] [Google Scholar]
- 28.Kim JR, Nosrat A, Fouad AF. Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J Dent. 2014:3. doi: 10.1016/j.jdent.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Wilson AD, Batchelor RF. Zinc oxide-eugenol cements: II. Study of erosion and disintegration. J Dent Res. 1970;49:593–8. doi: 10.1177/00220345700490032201. [DOI] [PubMed] [Google Scholar]


