Table 1. Optimization of the Direct Arylation of Complex 1a and 4-Iodotoluene (2a)a.
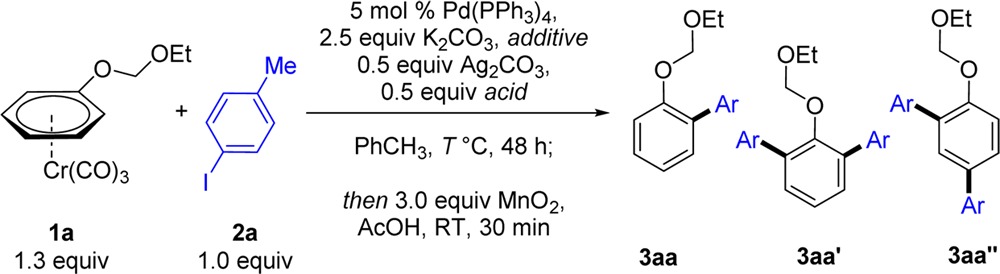
| entry | R–CO2H | additive (2 equiv) | T (°C) | 3aa:3aa′:3aa″ yield (%)b |
|---|---|---|---|---|
| 1 | 1-AdCO2H | – | 60 | 58:3:1 |
| 2 | PhCO2H | – | 60 | 34:1:1 |
| 3 | p-NO2–C6H4–CO2H | – | 60 | 0:0:0 |
| 4 | p-NMe2–C6H4–CO2H | – | 60 | 52:3:5 |
| 5 | 1-AdCO2H | piperidine | 60 | 0:0:0 |
| 6 | 1-AdCO2H | Et3N | 60 | 57:3:3 |
| 7 | 1-AdCO2H | TMP | 60 | 78:3:5 |
| 8 | 1-AdCO2H | TMP | 50 | 69:3:5 |
| 9c | 1-AdCO2H | TMP | 60 | 52:5:6 |
| 10 | – | TMP | 60 | 39:2:1 |
| 11d | 1-AdCO2H | TMP | 60 | 0:0:0 |
| 12e | 1-AdCO2H | TMP | 60 | 0:0:0 |
Reactions carried out on 0.1 mmol scale with respect to 2a.
Yield determined by 1H NMR of the crude using an internal standard.
No K2CO3 was added.
No Ag2CO3 was added.
20 equiv of (ethoxymethoxy)benzene were used instead of complex 1a.
