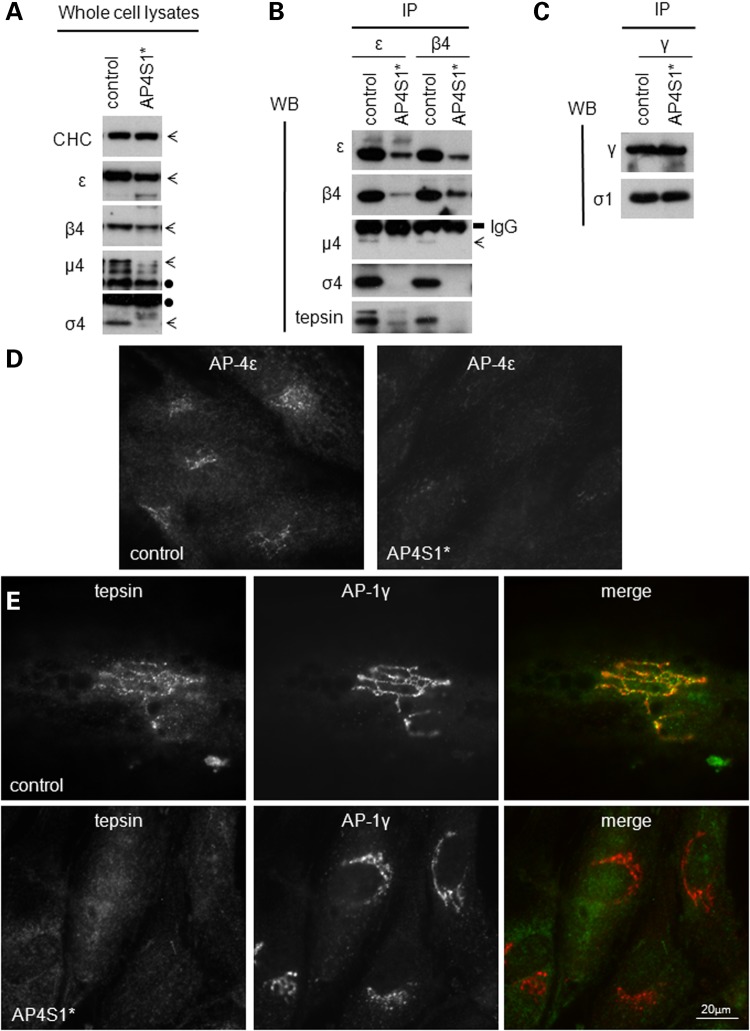
Figure 2.
Molecular characterization of the AP4S1 mutations described here. (A) Western blot of whole cell lysates show loss of σ4 and reduced levels of the other subunits in human fibroblasts from a patient with loss-of-function mutations in the AP4S1 gene (AP4S1*) versus a control sample. CHC was used as a loading control and did not show any differences. Bands corresponding to the different subunits are indicated with arrows, whereas cross-reaction bands are noted with a black dot. (B) Native immunoprecipitations (IPs) of control and patient cell lines were performed with antibodies against the β4 and ε subunit. Note the reduction of β4 and ε in their own IP, as well as the loss or reduction of all subunits, suggesting that the assembly of AP-4 is impaired. The interaction with the accessory protein tepsin is also clearly reduced in the AP-4-deficient patient. IgG bands confirm the use of equal amount of antibody in each IP. (C) Supernatant of the first IP (B) was used for a second IP with an antibody against AP-1γ. This shows no reduction of AP-1 complex assembly and is a control for equal amounts of starting material in the primary IP. (D and E) Results of immunofluorescence using an antibody against AP-4ε only (D) and double staining against tepsin and AP-1γ (E) on control- and patient AP4S1* patient-derived cell lines. The control line shows the punctuated perinuclear pattern typical for AP-4 localization. Whereas the patient line shows loss of AP-4 labeling (D) and loss of AP-4-associated tepsin (E). In contrast, the localization of AP-1γ is not affected in AP4S1* patient fibroblasts compared with the control.