Abstract
The complexity of cell interactions with their microenvironment and their ability to communicate at the autocrine, paracrine, and endocrine levels has gradually but significantly evolved in the last three decades. The musculoskeletal system has been historically recognized to be governed by a relationship of proximity and function, chiefly dictated by mechanical forces and the work of gravity itself. In this review article, we first provide a historical overview of the biomechanical theory of bone– muscle interactions. Next, we expand to detail the significant evolution in our understanding of the function of bones and muscles as secretory organs. Then, we review and discuss new evidence in support of a biochemical interaction between these two tissues. We then propose that these two models of interaction are complementary and intertwined providing for a new frontier for the investigation of how bone–muscle cross talk could be fully explored for the targeting of new therapies for musculoskeletal diseases, particularly the twin conditions of aging, osteoporosis and sarcopenia. In the last section, we explore the bone–muscle cross talk in the context of their interactions with other tissues and the global impact of these multi-tissue interactions on chronic diseases.
Keywords: Bones, Cross talk, Bone–muscle cross talk, Mechanostat, Muscles, Musculoskeletal, Myokines, Osteoporosis, Sarcopenia
Introduction
The complexity of cell interactions with their microenvironment and their ability to communicate at the autocrine, paracrine, and endocrine levels has gradually but significantly evolved in the last three decades. Until recently, adipose cells were considered to function primarily as storage compartments but are now beginning to be understood for their tremendous secretory capacity [1–4]. Another intriguing example comes from the gastrointestinal system. Our understanding of this system has advanced from viewing it merely as a site of digestion to one that secretes specific substances, cytokines, that modulate satiety, metabolism, and overall body weight [5]. These new discoveries of cytokines and signaling pathways placed in the context of systems biology provide new frontiers for investigating treatments for disorders that have proven intractable to current therapeutic approaches. The scientific community has enthusiastically embraced the ideas that the gastrointestinal, endocrine, and immune systems communicate at all of these levels. In light of the progress that is being made regarding systems interactions at the cellular level, it seems somewhat incongruent that the application of this same principle to other body systems has come much more slowly.
One such example of this slow progress is the musculoskeletal system, which has been historically recognized to be a relationship of proximity and function, chiefly dictated by mechanical forces and the work of gravity itself. The musculoskeletal system actually represents two-body systems that are closely related, not only anatomically and physiologically, but also pathophysiologically and with regard to the decline in function associated with aging. Increasing age is often accompanied by the progressive loss of muscle mass and strength, sarcopenia, and a decline in bone mass, osteoporosis. According to the 2010 Global Burden of Disease Study (GBD), musculoskeletal diseases are the second greatest cause of disability affecting nearly 2 billion people worldwide [6]. To a great extent, the way we see and have defined the musculoskeletal unit has created an axiom where the mechanical and physical interactions alone determine the fate of these tissues. When such a reasonable relationship between tissues is established and a solid flow of data continually supports that relationship, it all too easy for theories to become dogmas, thereby steering research toward one specific direction. Indeed, the celebrated acquisition of data supporting a particular physiologic or pathophysiologic perspective can obscure alternative views. Those associated with research and care of patients suffering gastrointestinal pathophysiology can certainly testify to the downside of such biomedical dogma as we all recall the 12-year struggle to accept H. pylori as the true cause of duodenal and gastric ulcers. Thankfully, such experiences teach us all to keep our minds open in an attempt to see as much of the physiologic picture as possible.
In this review paper, our goal is to discuss the emerging research that has recently been termed “bone–muscle cross talk,” which represents a departure from the traditional view of predominately mechanical interactions and proposes that bones and muscles also communicate biochemically. Particularly, we will focus our review on the paracrine and endocrine communication. To achieve this goal, we have structured our review article to address the following aspects of bone–muscle communication: (1) bones as biochemical communicators that secrete signaling factors, (2) muscles as biochemical communicators that secrete signaling factors, and (3) evidence of bone–muscle cross talk at the paracrine and endocrine levels. We will also explore the application of systems biology as well as the translational potential of this new knowledge, particularly for the treatment of the twin diseases of aging, osteoporosis and sarcopenia.
Bones as Biochemical Communicators
In the early 1900s, the prevailing view related to bone physiology was that osteoblasts promoted bone formation and osteoclasts promoted bone resorption. At the time, it was widely believed that hormones, dietary calcium, and other non-mechanical agents, all in an attempt to maintain bone homeostasis, primarily influenced the function of these two bone effector cells. Then, in the 1960s, that view was challenged as experts in bone physiology began differentiating between bone mineral density and bone strength. Interdisciplinary work from annual workshops hosted by the University of Utah initiated the development of an impressive body of evidence in support of the bio-mechanical relationship between bones and muscles. This new paradigm included the mechanostat model, a refinement of the nineteenth century Wolff's law, which purports that bone strength and density are largely a function of imposed mechanical forces [7]. That model, along with the Utah paradigm, continues to greatly influence investigations into bone physiology. A small part of that model, however, has seemingly been lost. Even Frost, the promoter of the biomechanical model, acknowledged the possible role of local and systemic non-mechanical agents effecting skeletal architecture. But, the biochemical aspect of the relationship between bones and muscles has not until more recently been explored to any great extent. Perhaps, this is due, in large part, to the need for a number of basic research advancements to first be developed, such as innovative techniques, cell lines, and equipment, as well as new knockout and transgenic animal models.
To our knowledge, one of the first lines of evidence that bones could function in an endocrine fashion was the suggestion in 1992 by Marotti et al. [8] that osteocytes might play a role in osteoblast modulation by way of gap junction signaling. Additional evidence of this suggestion was soon provided by elegant studies conducted by Tanaka et al. [9] demonstrating the production of soluble factors by osteocytes augmented osteoclastic development. At that same time, Klein-Nulend et al. [10] performed experiments that revealed the sustained release of prostaglandins from osteocytes following mechanical stimulation. And, in an attempt to explain how bone mass and structure is altered in response to mechanical load, Burger and Klein-Nulend [11] postulated the presence of cell signaling molecules as a key portion of the cellular mechanisms.
In 2003, Winkler et al. [12] provided evidence that osteocytes function as more than just a sensory cell, but also as a regulator of bone density through the secretion of sclerostin. Their postulations of dysregulation in bone formation resulted from the phenotypes observed in osteosclerosis patients and were further supported through genetic testing and the development of transgenic mice with increased sclerostin production and low bone mass. Since these early observations, work by a number of researchers including Bonewald, Johnson, Dallas, Karsenty, and Yamashita have continued to provide evidence in support of osteoblast/osteocyte-secreted factors that impact not only bone homeostasis but also distant tissues such as kidney, prostate, and brain as detailed below.
Yamashita and Shimata provided evidence of the physiological role of FGF23 in phosphate and vitamin D homeostasis as well as the pathophysiological role of FGF23 in osteomalacia [13]. In their 2012 review, Bonewald and Wacker [14] discussed FGF23 expression in osteocytes and its role in cardiovascular health. Although the exact pathways through which this occurs is not known at this time, evidence gained from transgenic mice phenotypes demonstrates that osteocyte expression of FGF23 is under the influence of molecules such as DMP1, PHEX, and MEPE [15].
Osteocalcin is a non-collagenous protein found in bone and dentin. In addition to providing structure, osteocalcin has been shown to have many functions, including energy metabolism, calcium ion homeostasis, and male fertility [16]. More than 20 years ago, bone cells were postulated to be the primary source of osteocalcin [17]; however, recent advances in genetic engineering have allowed deeper insight in support of this idea [16, 18–20]. In fact, osteocalcin along with other hormone-like substances secreted by bone cells is now thought to interact with substances from the liver and adipose tissue in a way that may predispose individuals to obesity, diabetes, non-alcoholic fatty liver disease, and osteoporosis. Prostaglandins are a class of naturally occurring lipid autacoids that derive from arachidonic acid and are produced by most cells, including bone cells. They have a wide range of functions, taking part in inflammation, pain mediation, smooth muscle contraction, and platelet aggregation. Prostaglandins also have been demonstrated to play a significant role in bone homeostasis, particularly the E and F series of prostaglandins [21, 22].
The growing list of bone cell-secreted factors is truly impressive and includes ATP, calcium, DKK1, DMP1, FGF23, Mepe, Nitric Oxide, OPG, osteocalcin, prostaglandins (particularly PGE2), RANKl, sclerostin, and Sost. These factors represent a myriad of biochemical structures ranging from simple organic molecules to complex proteins, which illustrates the plasticity of bone secretory capacity. Furthermore, the diversity of factors implies the role of bones in the modulation of the physiology of tissues throughout the body [23]. As our understanding of this new knowledge continues to develop and begins to be translated into meaningful and innovative therapeutic approaches, unprecedented advances will be achieved in the fight to constrain the epidemics of chronic diseases such as obesity, diabetes, osteoporosis, and sarcopenia.
Muscles as Biochemical Communicators
Skeletal muscle, so named for its functional connection and vicinity to the skeletal system, represents the largest organ in the body. The mechanical relationship between bones and muscles has been extensively studied, and it can be observed and understood in the context of the three major ontogenetic periods in the bone–muscle relationship—embryonic patterning, postnatal allometric growth, and the homeostatic relationship of adult life [24]. Bones and muscle cells not only share a common mesenchymal precursor, but also experience organogenesis through a tightly orchestrated network of genes during intrauterine development. It is understandable that the commonalities between these two tissues are reinforced through the mechanostat theory that postulates loads that create strains below a certain threshold stimulate bone loss through the inhibition of growth, while strains above a certain threshold stimulate growth and inhibit haversian remodeling [25]. Some researchers refer to the “bone–muscle unit” in deference to these observations that bones respond to varying levels of mechanical strain imposed by muscle mass and strength. The varying levels of mechanical strain appear to be modulated primarily by hormonal effects systemically, citing gender differences over time as evidence [26]. There is undeniably much evidence in support of the strong correlation between bone and muscle strength [27, 28].
Much has also been learned about the important relationship that exists between skeletal muscles and nerves, since motor neurons and the muscle fibers they innervate first came to be viewed as a single functional unit in the 1920s. After Henry Dale and Otto Loewi were awarded the 1936 Nobel Prize for their discoveries relating to the chemical transmission of nerve impulses, the body of knowledge in this area continued to grow leading to an enhanced understanding of differing muscle fiber types. The idea of the motor unit continues to be strengthened as instruments enabling molecular and genetic exploration to be undertaken. Investigations into muscle-to-nerve trophism led to the discovery of factors such as brain-derived neurotrophic factor (BDNF), NT-3 and NT-4/5 [29, 30]. Discoveries in these areas continue to provide hope of potential therapeutic targets for patients suffering from neuromuscular disorders such as the muscular dystrophies.
In the late 1970s, evidence emerged that skeletal muscle, as well as most tissues in the body, secret prostaglandins in response to injury, as Goldspink testified to the importance of skeletal musculature in terms of its metabolic effect on the body [31]. But it was only during the last decade that skeletal muscles became recognized more fully for their secretory capacity [32]. Pedersen et al. were the first to coin the term, “myokines,” after their discovery that contracting muscles not only secrete IL-6, but that it leads to a significant increase in IL-6 plasma levels. Building on earlier evidence from murine models that IL-6 is produced by myoblasts and myofibers in response to inflammation and injury, the Pedersen group showed that working muscles led to a 19-fold increase in arterial plasma IL-6 concentrations compared to resting muscle [33, 34]. This provided valuable evidence of skeletal muscle producing factors that impact not only tissues in close proximity, but also those at distant sites in the body. To support this, it was important to rule out that the IL-6 productions and secretion are not coming from immune cells. A notable observation has been made that with sepsis, there is an increase in TNFα, followed by an increase in IL-6. In sepsis, it appears that monocytes are the primary source of the increased TNFα. This is in contrast to the increase in IL-6 that accompanies exercise, as it is not preceded by TNFα [35]. Keller et al. demonstrated that the nuclear transcription rate of IL-6 increased markedly and rapidly with the onset of exercise [36]. Further evidence indicated that the IL-6 produced by exercising muscles impacted the output of hepatic glucose, thus adding strength to the premise that skeletal muscles do, indeed, function as endocrine organs.
The list of factors secreted from skeletal muscles continues to grow and includes IL-8, which has been shown to increase angiogenesis [37]; IL-5, which is an anabolic factor being investigated for its role in muscle–fat cross talk; IL-7, which is being studied for its impact on satellite cells during myogenesis [38] and brain-derived neutrophic factor (BDNF) [39]. Exercise has been found to induce a increase in mRNA of chemokine CXC motif ligand-1 (CXCL-1) aka KC (keratinocyte-derived chemokine) and a 2.4-fold increase in serum CXCL-1 [40]. Murine CXCL-1 is a functional homolog for IL-8 and belongs to a group that has gained attention for its role in inflammation, chemotaxis, angiogenesis, neuroprotective activity, and tumor growth regulation and is also associated with a decrease in visceral fat.
Most research associated with skeletal muscle secreted factors is in relation to factors produced in response to injury. IL-6 and LIF are produced and have been shown to enhance the myocyte differentiation after injury. Muscle regeneration is an ongoing phenomenon throughout the life span and provides an excellent opportunity for investigation into the endocrine function of this organ, as well as hope for targeted interventions to slow the process of muscle wasting. Two additional factors secreted by injured skeletal muscle are TGFα and TGFβ1 [32, 41]. These factors have an inhibitory effect on muscle cell proliferation and differentiation. It is believed that TGFβ1 triggers connective tissue proliferation and tissue fibrosis. The worldwide epidemics of obesity and type 2 diabetes continues to propel the concept that lack of exercise might favor an unbalance or reduced secretion of myokines, thereby contributing to these chronic diseases [40]. Last year, a new myokine brought hope for the development of molecules to target fat tissue accumulation, since irisin was shown to regulate the conversion of “bad” (white) fat into “good” (brown) fat that is essential for thermogenesis in mice [42]. Since the original publication, 49 papers have been published on the effects of irisin and a recent study by Park et al. [43] concluded that irisin might be directly associated with a higher risk of cardiovascular diseases and metabolic syndrome in humans, suggesting that augmented secretion of irisin by either adipocytes or muscle cells might occur to overcome an underlying irisin resistance.
Bone–Muscle Cross talk
How can endocrine properties of bone and muscle be physiologically relevant? The answer to this key question may lead to a bridge between the mechanical and biochemical theories. A feasible way of interpreting the role of these interactions is that they may serve to sense and transduce biomechanical signals such as unloading, loading, inactivity, or exercise, and even perhaps the translation of systemic hormonal stimulation into effective biochemical signals. Another way of interpreting and connecting these two theories is that one specific form of interaction could work as a priming for the other; in that, the physical effects of contraction on bone cells may prime these cells for the simultaneous, consecutive or ulterior effects of a secreted molecule (Fig. 1).
Fig. 1.
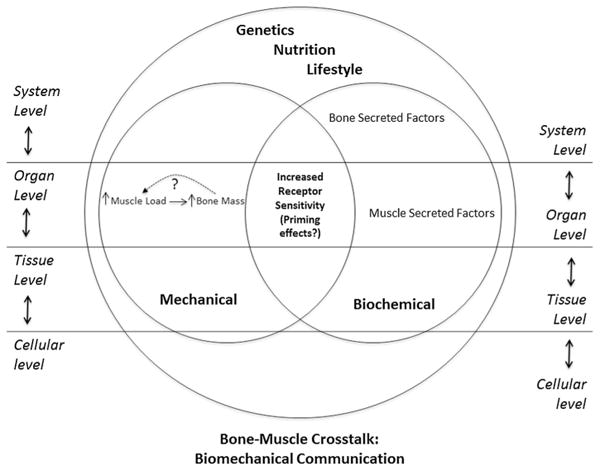
Proposed model of bone–muscle cross talk. This original drawing depicts both the mechanical and biochemical aspects of the bone–muscle cross talk. The largest circle in the figure represents the organismal level with the overarching influences of genetics, nutrition, and lifestyle. The two smaller circles represent the theories discussed in the article explaining bone–muscle communication. The area of overlap signifies that neither theory operates independent of the other. The “mechanical circle” presents the established principle that increased muscle load directly contributes to increased bone mass. The dotted line suggests a similar relationship in the reverse direction. The “biochemical circle” presents the growing body of evidence related to factors that are secreted from both bones and muscles. The central area of overlap labeled increased receptor sensitivity portrays the concept that both mechanical forces and secreted factors could prime or sensitize receptors in both tissues for reciprocal activity. This central area is also influenced by the global influences of genetics, nutrition, and lifestyle. On both sides of the figure, the interconnecting levels of organization reflect the influence of systems biology on the interpretation and understanding of bone-muscle cross talk
The close anatomical proximity of skeletal muscle and bone lends itself to hypothesize a relationship of paracrine nature, especially at the muscle fiber insertion sites along the periosteal interface. For evidence of such a relationship, we turn our attention to pathology and reflect upon conditions such as some of the bone stress syndromes where inflammation localized to the muscle area underneath the periosteal region spreads into the bone itself. These situations are consistent with the paracrine relationship hypothesis, suggesting inflammatory molecules from adjacent muscle fibers may penetrate into this region of the bone. Another powerful clinical example of this paracrine relationship is the application of muscle flaps around compounded bone fractures and their effects in promoting significantly faster healing for these fractured bones. Although the specific molecular mechanism of action is not completely understood, the introduction of muscle flaps has been used as a successful therapeutic approach to treat chronic osteomyelitis and to accelerate the healing of bone fractures [44]. It is possible that this same mechanism may be part of the process that occurs during bone and muscle healing after musculoskeletal injury. Studies performed by our group in osteocyte and muscle cell lines have determined that PGE2 secretion from osteocytes is more than 1,000 times larger than PGE2 secretion from muscle cells. This excess amount of PGE2 from osteocytes could interplay with injured muscles, which would aid in muscle regeneration and repair. Intriguingly, recent in vitro studies from our laboratory have provided support for a role of osteocyte-secreted PGE2 in aiding with the process of myogenesis [22].
To gain further insight into bone–muscle cross talk, we look to the phenotypic presentations of recently developed transgenic animal models. Myostatin was discovered in the late 1990s to be a potent inhibitor of muscle growth. It is expressed during development and in adult skeletal muscle, serving as an important negative regulator of skeletal muscle growth [45, 46]. Myostatin appears to decrease myoblast proliferation. The myostatin-deficient mouse model has increased muscle size and strength, with individual muscles weighing significantly more than wild-type mice [47]. Hamrick et al. used this myostatin-deficient mouse model to investigate the effects of increased muscle mass on bone mineral content and density. They found that although a consistent correlation was not found in all regions of the skeletal system, there was increased cortical bone mineral density in the distal femur and an increased periosteal circumference along the humerus [48–50]. Another group used the same myostatin-deficient mouse model to look at the impact of the chronic loss of myostatin on multiple organ systems and found that it appeared to preserve bone density [51]. From a contrasting perspective, Zimmers [47] investigated the effects of myostatin over-expression in an animal model and observed a profound loss of muscle and fat, mimicking the presentation seen in chronically ill patients and commonly referred to clinically as cachexia. The authors encourage further research into the disruption of myostatin in an effort to preserve muscle mass in patients with chronic diseases.
As mentioned above, osteocalcin serves as a splendid example of the endocrine function of bone cells [23]. The circulating level of this osteoblast-derived factor increases with exercise, binds to the Gprc6a receptor, and affects distant adipocytes and β cells of the pancreas. Perhaps to balance the physiologic scales, osteoblasts also naturally express the osteotesicular phosphatase gene (Esp), which inhibits the function of osteocalcin [52]. With this information in mind, it is of specific interest to the discussion of bone–muscle cross talk that Gprc6a knockout mice display the phenotype of decreased muscle mass, while Esp knockout mice have increased muscle mass. Through these observations, it can be proposed that osteocalcin, a known bone cell factor, may play a role in the regulation of muscle mass. Certainly, this new knowledge could contribute to a deeper understanding of sarcopenia, and this osteoblast-derived factor could be a target for the development of therapies to prevent, delay, or slow the progression of this highly prevalent disorder associated with aging. If this is useful for sarcopenia, it is possible that it may also be useful for its associated disorder, osteoporosis. The endocrine communication that continues to be revealed through the effects of myokines and osteokines is not limited merely to a bone–muscle connection. As illustrated in Fig. 2, there is increased awareness that the factors secreted from tissues throughout the body impact the overall health of the individual. Support of this dynamic interrelationship continues to build, especially with regard to the development of chronic diseases such as obesity, diabetes, and metabolic syndrome. Just as with sarcopenia and osteoporosis, all of these diseases are on the rise in the elderly population. According to the Centers for Disease Control and Prevention, the prevalence of obesity among American adults 65 years of age and older is nearly 35 %, translating into more than 8 million older adults [53]. The American Diabetes Association Web site reports that nearly 25 % of adults aged 60 and over have diabetes, and it is also becoming clear that metabolic syndrome prevalence increases with age [54]. Recognizing the magnitude of the public health problems posed by sarcopenia, and knowing skeletal muscles to be much more than contractile motors, a review authored by a distinguished team of researchers [55] called for increased research into the factors involved in the pathogenesis of sarcopenia more than a decade ago, in 2001. Data from many studies published around that same time began to suggest that sarcopenia impacted the development of other chronic conditions such as cardiovascular and metabolic diseases. These researchers were observing that sarcopenia apparently leads to dyslipidemia, insulin resistance, and hypertension as well as a decline in immunologic function [56, 57], which one would predict or expect since muscles are quintessential for overall body metabolism. A consistent clinical observation is that cachexia is a direct cause of death as recently reviewed and even suggested by the title of the article by Kalantar-Zadeh et al. [58].
Fig. 2.
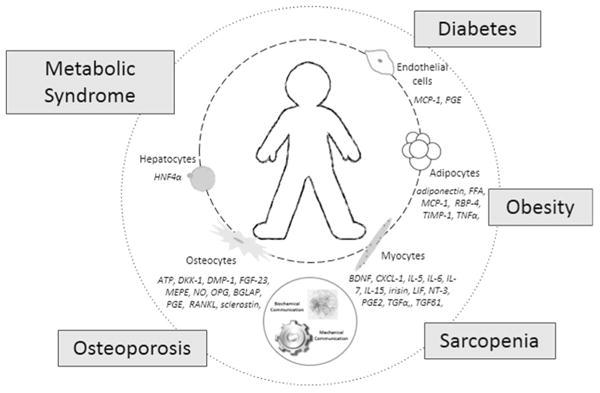
Bone–muscle cross talk, interactions with other tissues, and the impact on chronic diseases. This original drawing illustrates the concept that interactions among different tissues throughout the organism are abundant and much more complex than previously realized. In this larger context, bone–muscle cross talk remains both physiologically and pathologically relevant but is also seen as being affected by other tissues of the body. At the center of this figure is the outline of an individual, the patient. The smaller circle, closest to the patient, lists cells discussed in the text, along with factors they are known to secrete. The dashed line connecting these cells indicates that they are connected biochemically through the impact that their secreted factors have on one another. The larger circle surrounding the patient lists a number of conditions and diseases impacted by the biochemical interactions between cells listed and others. Special significance for multi-tissue/organ cross talk is revealed by pathological conditions such as obesity, diabetes, and metabolic syndrome. The dotted line of this larger circle indicates the developing understanding that these conditions and diseases impact one another. These conditions seem to directly influence sarcopenia and osteoporosis as detailed in the text
More than a decade ago, Baumgartner [59] observed that many older adults with sarcopenia were also obese, with the prevalence increasing with age. The sarcopenicobese older adult drew the attention of his research team during the New Mexico Aging Process Study, because they found this subsector of the elderly population to be at especially high risk of physical disability, balance and gait problems, and falls. In that study, Baumgartner defined sarcopenia as muscle mass more than two standard deviations (SD) below the mean relative skeletal muscle mass in a healthy, younger person. That was the first time muscle mass was used as the published criterion for sarcopenia. Obesity was defined as percent body fat>27 % in men and 38 % in women. One of the remarkable observations this group made caused them to propose that many obese individuals convert to sarcopenic-obesity with increasing age due to the loss of lean muscle mass. Jensen and Friedmann [60] reported similar findings of an increased risk among obese older adults. Intriguingly, we still do not know the exact role or functions of lean muscle mass over fatty muscle mass, but the link between better health and lean muscle mass is undisputable.
Additional health risks observed in sarcopenic older adults include insulin resistance and the development of type 2 diabetes mellitus. Srikanthan et al. [61] conducted a study to investigate the relationship between sarcopenia, obesity, and age-related insulin resistance. In their cross-sectional analysis of the National Health and Nutrition Examination Survey III (NANES III), they concluded that sarcopenia, independent of obesity, is associated with compromised glucose metabolism. Another study conducted around the same time concurred that type 2 diabetes was associated with an increased risk of sarcopenia [62]. Certainly, a relationship between diabetes and sarcopenia makes physiologic sense from the perspective that skeletal muscle represents the largest single sinker for blood glucose. A decline in muscle mass with aging is, therefore, associated with a decrease in sites for glucose uptake, which will be further exacerbated by the decline in physical activity. At the same time, there is a decrease in glycogen synthesis, which might be a factor limiting longer bouts of physical activity, contributing to the increased fatigability reported in older adults. Along with this, data support an increase in triglycerides with aging, which have been indicted both in age-related mitochondrial damage and with blocking of ability of insulin to facilitate glucose entry into the cell. All of these phenomena contribute to an increase in blood glucose. Insulin may play a significant role in all of this, as it is a potent anabolic hormone that impacts glucose, protein, and lipid metabolism. It facilitates glucose uptake, inhibits hepatic glucose uptake and triglyceride production, inhibits skeletal muscle protein synthesis, and inhibits adipose tissue lipolysis [63]. Recognizing this relationship, a recent study provides data supporting a direct relationship between insulin resistance, the loss of lean muscle mass, and the gain of fat mass in men aged 65 and older [64]. The chronic complications of diabetes mellitus impact systems throughout the body, including bones. Individuals with type 1 diabetes mellitus have lower bone mass density, with impaired bone formation believed to be the primary cause [65]. Patients with either type 1 or type 2 diabetes experience hypercalciuria during times of glycosuria. This increased loss of calcium has been hypothesized to contribute to impaired bone quality observed with diabetes, although the direct effects of this loss of calcium on skeletal muscle function remain elusive. Interestingly, patients with type-2 DM have an increased BMD, but also have an increased risk of bone fragility [66, 67]. Although through different mechanisms, both type 1- and type 2-DM predispose patients to osteo-porotic fractures [68]. It is likely that the increased BMD in type-2 DM reflects a compensatory mechanism that is not present or lost in type-1 DM.
Clinicians now agree that metabolic syndrome is a valid diagnosis in individuals with at least three for the following signs: waist circumference ≥40 in. (men) or ≥35 in. (women); triglycerides ≥150 mg/dl; HDL <40 mg/dl (men) or <50 mg/dl (women); blood pressure ≥130/85; and blood glucose >110 mg/dl. The presence of metabolic syndrome identifies a group of overweight or obese patients at significant risk for insulin resistance, ectopic fat accumulation, and cardiovascular and other diseases. With the rapid increase in the number of older adults, along with the rise in obesity, diabetes, sarcopenia, and osteoporosis, research into the connection between all of these conditions is warranted, and tissue cross talk from both a physical and a biochemical point view should be a leading force toward new advancements (Fig. 2).
Future Directions
To build on the growing body of knowledge related to bone–muscle interactions, we recommend continued research into both the mechanical relationship and biochemical cross talk that exists. The challenge is to accomplish this while avoiding the trap of false dichotomies. The time is here for integrated research that utilizes the new tools of systems biology to answer the fundamental questions of how mechanical and biochemical communication are part of the overall bone–muscle cross talk. Missing at this point are highly specific bone–muscle models, to allow manipulation of selected genes and factors, both biochemical and mechanical, and observe their impact on the bone–muscle unit. The third challenge will be the expansion of this work to include three additional participants in the bone–muscle unit: cartilage, ligaments, and tendons. Additionally, and in concert with these recommended investigations, we recommend continued research into the relationships that exist between sarcopenia, osteoporosis, and other chronic diseases such as obesity, diabetes, and other disorders of energy metabolism. While advances are being made in these areas, it will be ever more important to bridge the gap between bench research and clinical practice to assure that contributions to the body of knowledge truly translate into meaningful therapeutic approaches and interventions for patients with musculoskeletal diseases and also for humankind, at the level of prevention.
Acknowledgments
This work was supported by the NIH-National Institutes of Aging Program Project Grant P01 AG039355-01-A1, Missouri Life Sciences Research Board and the Thompson Endowment Fund (MB). We are thankful to the members of the UMKC Bone Biology Group and Muscle Biology Group for useful discussions that were helpful in the preparation of this manuscript.
Footnotes
Disclosures: Conflict of interest: Janalee Isaacson and Marco Brotto declare that they have no conflict of interest.
Animal/Human Studies: This review does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Janalee Isaacson, Muscle Biology Research Group-MUBIG, School of Nursing and Health Studies, University of Missouri-Kansas City (UMKC), 2464 Charlotte St, Kansas City, MO 64108, USA; Nursing Program, Johnson County Community College, Overland Park, KS 66210, USA.
Marco Brotto, Email: brottom@umkc.edu, Muscle Biology Research Group-MUBIG, School of Nursing and Health Studies, University of Missouri-Kansas City (UMKC), 2464 Charlotte St, Kansas City, MO 64108, USA; School of Medicine, UMKC, Kansas City, MO, USA; School of Pharmacy, UMKC, Kansas City, MO, USA.
References
- 1.Ioannidis I. The road from obesity to type 2 diabetes. Angiology. 2008;59(Supp 2):39S–43S. doi: 10.1177/0003319708318583. [DOI] [PubMed] [Google Scholar]
- 2.Berg AH, et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 3.Sell H, Dietze-Schroeder D, Eckel J. The adipocyte–myocyte axis in insulin resistance. Trends Endocrinol Metab. 2006;17(10):416–22. doi: 10.1016/j.tem.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Lafontan M. Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways. AJP Cell Physiol. 2011;302:C327–59. doi: 10.1152/ajpcell.00168.2011. [DOI] [PubMed] [Google Scholar]
- 5.Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. 2013;9:584–97. doi: 10.1038/nrendo.2013.136. [DOI] [PubMed] [Google Scholar]
- 6.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years live with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost HM. Perspectives: a proposed general model of the “mechanostat” (suggestions from a new skeletal-biologic paradigm) Anat Rec. 1996;244:139–47. doi: 10.1002/(SICI)1097-0185(199602)244:2<139::AID-AR1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 8.Marotti G, Ferretti M, Muglia MA, Palumbo C, Palazzini S. A quantitative evaluation of osteoblast–osteocyte relationships on growing endosteal surface of rabbit tibiae. Bone. 1992;13:363–8. doi: 10.1016/8756-3282(92)90452-3. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Matsuo T, Ohta M, Sato T, Tezuka K, Nijweide PJ, et al. Time-lapse microcinematography of osteocytes. Miner Electrolyte Metab. 1995;21:189–92. [PubMed] [Google Scholar]
- 10.Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASB J. 1995;9:441–5. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 11.Burger EH, Klein-Nulend J. Mechanotransduction in bone—role of the lacuna-canalicular network. FASEB J. 1999;13:101–12. [PubMed] [Google Scholar]
- 12.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of one formatin via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiologyical role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonewald LF, Wacker MJ. FGF23 production by osteocytes. Pediatr Nephrol. 2012;28:563–8. doi: 10.1007/s00467-012-2309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–62. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 17.Lajeunesse D, Kiebzak GM, Frondoza C, Sacktor B. Regulation of osteocalcin secretion by human primary bone cells and by the human osteosarcoma cell line MG-63. Bone Miner. 1991;14:237–50. doi: 10.1016/0169-6009(91)90025-u. [DOI] [PubMed] [Google Scholar]
- 18.Ducy P, Desbois C, Boyce B, Pinero G, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 19.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agas D, Marchetti L, Hurley MM, Sabbieti MG. Prostablandin F2α: a bone remodeling mediator. J Cell Physiol. 2013;228:25–9. doi: 10.1002/jcp.24117. [DOI] [PubMed] [Google Scholar]
- 22.Mo C, Romero-Suarez S, Brotto MA. Pge2 accelerates myogenesis of C2C12 myoblasts. Biophys J. 2011;100:288a. [Google Scholar]
- 23.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–20. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orestes-Cardoso SM, Nefussi JR, Hotton D, Mesbah M, Orestes-Cardoso MDS, Robert B, et al. Postnatal Msx1 expression pattern in craniofacial, axial, and appendicular skeleton of transgenic mice from the first week until the second year. Dev Dyn. 2001;221:1–13. doi: 10.1002/dvdy.1120. [DOI] [PubMed] [Google Scholar]
- 25.Pearson OM, Lieberman DE. The aging of Wolff's law: ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol. 2004;125:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 26.Lang TF. The bone–muscle relationship in men and women. J Osteoporos. 2011;2011:1–4. doi: 10.4061/2011/702735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanchetta JR, Plotkin H, Filgueira MLA. Bone mass in children: normative values for the 2–20-year-old population. Bone. 1995;16:S393–9. doi: 10.1016/8756-3282(95)00082-o. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Alen M, Nicholson P, Suominen H, Koistinen A, Kroger H, et al. Weight-bearing, muscle loading and bone mineral accrual in pubertal girls—a 2-year longitudinal study. Bone. 2007;40:1196–202. doi: 10.1016/j.bone.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 29.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–38. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Pinilla F. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–95. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 31.Goldspink DF, Goldspink G. The role of passive stretch in retarding muscle atrophy. In: Nix WA, Vrbova G, editors. Electrical stimulation and neuromuscular disorders. Berlin: Springer; 1986. pp. 91–100. [Google Scholar]
- 32.Kurek JB, Bower JJ, Romanella M, Koentgen F, Murphy M, Austin L. The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve. 1997;20:815–22. doi: 10.1002/(sici)1097-4598(199707)20:7<815::aid-mus5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24:113–9. doi: 10.1023/a:1026070911202. [DOI] [PubMed] [Google Scholar]
- 34.Steensberg A, Hall G, Osada T, Sacchetti M, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–42. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersen K, Pedersen B. The role of inflammation in vascular insulin resistance with focus on IL-6. Horm Metab Res. 2008;40:635–9. doi: 10.1055/s-0028-1083810. [DOI] [PubMed] [Google Scholar]
- 36.Keller C. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15(14):2748–50. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen AR, Pedersen BK. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl Physiol Nutr Metab. 2007;32:833–9. doi: 10.1139/H07-054. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen BK, Akerstrom TCA, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–8. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 39.Matthews VB, Astrom MB, Chan MHS, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–18. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen L, Olsen CH, Pedersen BK, Hojman P. Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. AJP Endocrinol Metab. 2012;302:E831–40. doi: 10.1152/ajpendo.00339.2011. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161:895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seale P, Bjork B, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hee Park K, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(12):4899–907. doi: 10.1210/jc.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan JKK, Harry L, Williams G, Nanchahal J. Soft-tissue reconstruction of open fractures of the lower limb: muscle versus fasciocutaneous flaps. Plast Reconstr Surg. 2012;130:284e–95e. doi: 10.1097/PRS.0b013e3182589e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 46.Jouliaekaza D, Cabello G. The myostatin gene: physiology and pharmacological relevance. Curr Opin Pharmacol. 2007;7:310–5. doi: 10.1016/j.coph.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Zimmers TA. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–8. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 48.Hamrick MW, McPherron AC, Lovejoy CO. Bone mineral content and density in humerus of adult myostatin-deficient mice. Calcif Tissue Int. 2002;71:63–8. doi: 10.1007/s00223-001-1109-8. [DOI] [PubMed] [Google Scholar]
- 49.Hamrick MW. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec. 2003;272A:388–91. doi: 10.1002/ar.a.10044. [DOI] [PubMed] [Google Scholar]
- 50.Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2005;21:477–83. doi: 10.1359/JBMR.051203. [DOI] [PubMed] [Google Scholar]
- 51.Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, et al. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8:573–83. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coiro V, Volpi R, Cataldo S, Magotti MG, Maffei ML, Giumelli C, et al. Effect of physiological exercise on osteocalcin levels in subjects with adrenal incidentaloma. J Endocrinol Invest. 2012;35:357–8. doi: 10.1007/BF03345430. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (US) Prevalence of obesity among older adults in the United States, 2007–2010. 2012 NCHS Data Brief; no. 106. [Google Scholar]
- 54.Beltran-Sanchez H, Harhay MO, Harhay MM, McElliogott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62(8):697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 56.Karakelides H, Nair KS. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123–48. doi: 10.1016/S0070-2153(05)68005-2. [DOI] [PubMed] [Google Scholar]
- 57.Cosqueric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96(5):895–901. doi: 10.1017/bjn20061943. [DOI] [PubMed] [Google Scholar]
- 58.Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle. 2013;4:89–94. doi: 10.1007/s13539-013-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 60.Jensen GL, Friedmann JM. Obesity is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc. 2002;50(5):918–23. doi: 10.1046/j.1532-5415.2002.50220.x. [DOI] [PubMed] [Google Scholar]
- 61.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5(5):1–7. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes. Diabetes Care. 2010;33(7):1497–9. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition. 2010;26(7–8):686–93. doi: 10.1016/j.nut.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc. 2011;59:1217–24. doi: 10.1111/j.1532-5415.2011.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hofbauer LG, Brueck C, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. JBMR. 2007;22(9):1317–28. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- 66.Strotmeyer ES, Cauley JA. Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes. 2007;14:429–35. doi: 10.1097/MED.0b013e3282f1cba3. [DOI] [PubMed] [Google Scholar]
- 67.Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009;84:45–55. doi: 10.1007/s00223-008-9195-5. [DOI] [PubMed] [Google Scholar]
- 68.Hamann C, Kirschner S, Gunther KP, Hofbauer LC. Bone sweet bone—osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8:297–305. doi: 10.1038/nrendo.2011.233. [DOI] [PubMed] [Google Scholar]


