Abstract
We have shown previously the oncolytic potential of myxoma virus in a murine xenograft model of human glioma. Here, we show that myxoma virus used alone or in combination with rapamycin is effective and safe when used in experimental models of medulloblastoma in vitro and in vivo. Nine of 10 medulloblastoma cell lines tested were susceptible to lethal myxoma virus infection, and pretreatment of cells with rapamycin increased the extent of in vitro oncolysis. Intratumoral injection of live myxoma virus when compared with control inactivated virus prolonged survival in D341 and Daoy orthotopic human medulloblastoma xenograft mouse models [D341 median survival: 21 versus 12.5 days; P = 0.0008; Daoy median survival: not reached (three of five mice apparently “cured” after 223 days) versus 75 days; P = 0.0021]. Rapamycin increased the extent of viral oncolysis, “curing” most Daoy tumor-bearing mice and reducing or eliminating spinal cord and ventricle metastases. Rapamycin enhanced tumor-specific myxoma virus replication in vivo and prolonged survival of D341 tumor-bearing mice (median survival of mice treated with live virus (LV) and rapamycin, versus LV alone, versus rapamycin alone, versus inactivated virus: 25 days versus 19, 13, and 11 days, respectively; P < 0.0001). Rapamycin increased the levels of constitutively activated Akt in Daoy and D341 cells, which may explain its ability to enhance myxoma virus oncolysis. These observations suggest that myxoma virus may be an effective oncolytic agent against medulloblastoma and that combination therapy with signaling inhibitors that modulate activity of the phosphatidylinositol 3-kinase/Akt pathway will further enhance the oncolytic potential of myxoma virus.
Introduction
Medulloblastoma is the most common malignant brain tumor in children. It is difficult to treat because tumor cells invade the surrounding normal tissue and disseminate through the cerebral spinal fluid, leading to metastasis throughout the cerebral spinal axis. Intensive multimodality treatment that combines surgery, craniospinal irradiation, and multiple drug chemotherapy can achieve long-term survival rates of 50% to 70%, but at significant cost due to toxic side effects (1–3). Most medulloblastoma survivors suffer long-term treatment-related morbidity that greatly affects their quality of life (1, 2). New therapeutic approaches with superior efficacy and reduced toxicity are urgently needed in the clinical management of children with medulloblastoma (3, 4).
Oncolytic viruses are able to selectively infect and kill tumor cells by exploiting the same cellular defects that promote tumor growth (for review, see ref. 5). For example, many tumor cells acquire mutations that allow them to escape IFN-mediated signaling pathways that regulate proliferation and apoptosis, but defects in IFN signaling also cripple the innate antiviral response of tumor cell. Tumor cells often have defects in translational control or in other signaling pathways, such as those involving Myc, Ras, Akt, and p53, which render them susceptible to lethal viral infections. An expanding number of oncolytic viruses have shown promising results in experimental brain tumor models, and a few have been tested in clinical trials with modest success (5–10). Two studies examined the efficacy of oncolytic virotherapy in medulloblastoma. A mutant adenovirus, Delta-24, was shown to infect and kill human medulloblastoma cell lines in culture (11). We also showed that reovirus was able to kill human medulloblastoma cell lines and tumor specimens in vitro and that intratumoral (i.t.) injection of reovirus in an orthotopic mouse model of human medulloblastoma significantly prolonged survival and reduced the frequency of metastases (12). These results are encouraging and suggest that one or more oncolytic viruses might prove to be effective novel therapies against medulloblastoma.
Ideally, an oncolytic virus should be able to selectively infect and kill tumor cells while sparing normal nontransformed cells. Other desirable properties of oncolytic viruses include the ability to engineer the virus (to increase efficacy and selectivity) and a nonpathogenic profile in humans. Myxoma virus is a rabbit-specific poxvirus that is considered a promising oncolytic virus platform. Its tropism is normally highly restricted to European rabbits, and it is nonpathogenic for all other vertebrate species tested, including humans (13). Despite this narrow specificity, myxoma virus is capable of infecting and killing a wide variety of human tumor cell lines (7, 14). We have shown the safety and oncolytic potential of myxoma virus in a murine xenograft model of human malignant glioma (7). Myxoma virus is amenable to genetic engineering because its large dsDNA genome is fully sequenced (15) and can accommodate a large amount of foreign gene material (16, 17). Although we do not have a complete understanding of all the underlying signaling defects that cause tumor cells to become susceptible to myxoma virus, we have found that high endogenous levels of phosphorylated Akt in human cancer cells indicate susceptibility to myxoma virus infection and that constitutively activation of Akt is required for productive myxoma virus infection (18). Indeed, the M-T5 gene product from MV acts as a functional homologue of PIKE-A, a cellular activator of Akt (19).
The molecular pathogenesis of medulloblastoma is only partially understood, but aberrant signaling through several different pathways is involved, including pathways that lead to activation of phosphatidylinositol 3-kinase (PI3K) and its downstream effector Akt (3, 20, 21). The frequency of Sonic hedgehog (Shh)– induced medulloblastoma in mice is significantly increased by activation of the PI3K/Akt signaling pathway (22). Evidence of phosphorylated Akt and PTEN mutations in human medulloblastoma samples suggests that constitutive activation of the PI3K/Akt pathway is an important step in medulloblastoma tumorigenesis (21). Overexpression of several different growth factor receptors, including insulin-like growth factor-I (IGF-I) receptor, also contributes to medulloblastoma tumorigenesis, in part by activating PI3K (23). The serine/threonine kinase mammalian target of rapamycin (mTOR) is one important downstream target of Akt. Upon activation, mTOR acts as a central modulator of cell proliferation in medulloblastoma (23, 24). Therefore, modulation of the PI3K/Akt/mTOR signaling pathway is a potential therapeutic strategy against medulloblastoma.
Inhibitors of mTOR, such as rapamycin, RAD001, and CCI-779, have been evaluated as novel agents for brain tumor therapy (25–30). However, rapamycin alone produces only partial responses in medulloblastoma (26) and some brain tumor cell lines are resistant to rapamycin (27, 28). CCI-779 had only limited antitumor activity as a single agent against recurrent glioblastomas in phase II studies (29, 30). Some studies have shown increased efficacy when oncolytic viruses were used in combination with chemotherapy drugs (31, 32). Pretreatment with rapamycin increased levels of activated Akt as well as myxoma virus tropism and spread in vitro in human tumor cell lines that are ordinarily nonpermissive (33). It is possible that combination therapy using mTOR inhibitors and oncolytic viruses could be more effective than using either agent alone.
Here, we evaluated the potential of myxoma virus as an oncolytic agent against medulloblastoma in vitro and in vivo in orthotopic human medulloblastoma mouse models. The potential therapeutic synergism of combined treatment with myxoma virus and rapamycin was also investigated using both in vitro and in vivo approaches.
Materials and Methods
Cell lines
Murine NIH3T3 fibroblasts and the human medulloblastoma cell lines Daoy, UW228, TE671, D283, and D341 were obtained from the American Type Culture Collection. Human medulloblastoma lines D425 and D384 were supplied by Dr. Darrell Bigner (Duke University, Durham, NC). Two murine medulloblastoma cell lines, Ptc850 and Ptc1102, were derived from spontaneous medulloblastoma arising in Ptc1+/− mice (34, 35). ONS76 cells were obtained from the Institute for Fermentation, Osaka Animal Cell Bank (Osaka, Japan). Cells were grown in DMEM/F12 containing 10% (Daoy, UW228, TE671, NIH3T3, Ptc850, and Ptc1102) or 20% (D341, D384, D283, and D425) fetal bovine serum (FBS) at 37°C in a humidified 5% CO2 incubator. Each cell line was tested routinely for Mycoplasma contamination.
Viruses
Two derivatives of myxoma virus (strain Lausanne), created by intergenic insertion of a marker cassette, were used for infection studies: vMyxgfp, which contains a green fluorescent protein (GFP) cassette driven by a synthetic vaccinia virus early/late promoter, and vMyxlac, which contains a β-galactosidase cassette driven by a late MV promoter. Both viruses were propagated and titrated by focus formation on BGMK cells as described previously (36). UV-inactivated (“dead”) myxoma virus (DV) was prepared by irradiating virus with UV light for 2 h.
Cell infection in vitro
Cells were seeded to 70% to 80% confluence in 96-well plates before treatment with virus and/or rapamycin (LC Laboratories). Control cells included cells that were not exposed to virus or rapamycin, as well as separate samples treated with DV. Cells were incubated at 37°C for 1 h in 50μL of serum-free medium containing the indicated multiplicity of infection (MOI) of live or UV-inactivated myxoma virus, after which 150 μL of fresh medium were added to each well and cells were cultured until needed for further analysis. For the combined therapy group, rapamycin was added to the cells 1 h before treatment with virus. Phase-contrast and fluorescent images of cells were taken using a Carl Zeiss inverted microscope (Axiovert 200M) mounted with Carl Zeiss digital camera (AxioCam Mrc).
Transfection of medulloblastoma cells with GFP
Daoy cells were transfected with the GFP expression plasmid using Fugene transfection reagent (Roche Diagnostic Co.). A mixture of Fugene and plasmid DNA was incubated for 30 min at room temperature in serum-free culture medium. The DNA mixture was applied to Daoy cells for 4 h at 37°C in serum-free medium and then FBS was added to a final concentration of 10%. Cells were cultured for 4 days and the culture medium was changed daily. After 4 days, transfected cells were selected using G418 antibiotic (200 μg/mL), and transfected clones were identified by fluorescent microscopy to detect GFP expression. GFP expression was detected in >95% of the cells, as determined by flow cytometry.
In vitro analysis of cytopathic effect and viral infection
Cells were seeded at 5 × 104 cells per well in six-well plates and cultured overnight. After infection with vMyxgfp, vMyxlac, or DV at a MOI of 5 for 48 h, cells were imaged under phase contrast and by immunofluorescence. vMyxlac-infected cells were stained with X-gal to detect β-galactosidase activity. Cells were washed with PBS, fixed for 10 min in acetone, incubated for 4 to 8 h at room temperature with X-gal staining solution (100 μg/mL X-gal, 500 μmol/L potassium ferrocyanide, and 200 μmol/L MgCI2 in PBS), and then photographed under phase contrast.
Cell viability assays
Cells grown to 70% confluence were infected with different doses (MOI of 0, 1, and 10) of vMyxgfp in the presence or absence of rapamycin (20 or 100 nmol/L). Cell viability was measured 72 h after infection using the 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) assay as described (37).
Immunohistochemistry for viral protein expression
Frozen sections were fixed with 4% paraformaldehyde for 20 min, followed by three washes with PBS. The sections were exposed to the primary antibody (M-T7; rabbit polyclonal anti-myxoma serum diluted 1:2,000 in PBS containing 2% bovine serum albumin; refs. 7, 38) for 12 to 16 h at 4°C. Biotinylated anti-rabbit IgG (Vector Laboratories) was used as a secondary antibody and the ABC Immunohistochemistry kit (Vector Laboratories) was used for immunostaining.
Western blotting
Cells were infected with 5 MOI of vMyxgfp with or without rapamycin (20 nmol/L) as described above. Forty-eight hours later, cells were washed with PBS and lysed. Protein concentrations of cell lysates were measured using the bicinchoninic acid protein assay (Biolynx, Inc.). Protein samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. Blots were probed with antibodies recognizing the viral proteins M-T7 and Serp-1 (7, 38), phosphorylated and total Akt (Cell Signaling Technology), and phosphorylated and total 70-kDa ribosomal protein S6 kinase (p70S6K; Cell Signaling Technology). Immunoreactive proteins were detected by enhanced chemiluminescence. Blots were probed with an anti– β-actin antibody (Sigma) to confirm equal protein loading.
Virus recovery assay
Medulloblastoma cell lines were seeded at density of 5 × 104 cells per well in six-well plates. Twenty hours later, cells were treated with vMyxgfp alone [MOI of 0.1 plaque-forming unit (PFU) per cell] or with vMyxgfp and 20 nmol/L rapamycin in serum-free medium for 1 h at 37°C. Virus-containing medium was removed, replaced with fresh medium, and cells were cultured until the designated time points. Plates were then subjected to three rounds of freezing and thawing to release intracellular viral particles. Serial dilutions of supernatants and cell lysates were cultured on confluent layers of BGMK cells and viral titers were determined by counting fluorescent viral foci 48 h later. All samples were tested and experiments were replicated in triplicate.
Animals
CD-1 nude mice (female, 6–8 weeks old) were purchased from Charles River Canada. The animals were housed in groups of three to five in a vivarium maintained on a 12-hour light/dark schedule with a temperature of 22 ± 1°C and a relative humidity of 50 ± 5%. Food and water were available ad libitum. All procedures were reviewed and approved by the University of Calgary Animal Care Committee.
In vivo studies in CD-1 nude mice bearing Daoy medulloblastoma tumors
An orthotopic animal model was established with the human medulloblastoma cell line Daoy. The stereotactic techniques used to implant medulloblastoma cells (1 × 105 cells per mouse) in the cerebellum have been described previously (7, 37). Twelve or 25 days later, an i.t. injection of 5 × 106 PFUs of live vMyxgfp or DV in 2 μL PBS was administered stereotactically over 10 to 20 s. The “late-stage” model (with treatment beginning after 25 days) was injected every other day for a total of three injections. Animals losing 20% or more of their body weight or having trouble ambulating, feeding, or grooming were sacrificed. Animals were anesthetized, perfused intracardially with PBS, and then fixed using 4% paraformaldehyde. Brains and major organs were examined histologically.
To test the effect of combination therapy using myxoma virus and rapamycin, tumor-bearing animals were divided into the following four treatment groups (nine animals per group) 11 days after implantation of Daoy-GFP cells: (a) DV control, (b) rapamycin (5 mg/kg, five times weekly for a total of 2 weeks), (c) vMyxgfp (5 × 106 PFUs/mouse/injection, every other day for a total of three injections), and (d) vMyxgfp and rapamycin. Rapamycin was administered i.p. beginning 11 days after tumor implantation, whereas vMyxgfp injections began after 12 days. To assess metastasis, three animals from each group were sacrificed 30 days after the last virus injection. The brain and spinal cord were removed, photographed using a fluorescent stereotactic microscope to count GFP-expressing metastases, and then completely serially sectioned and examined by fluorescent microscopy. The remaining six animals in each group were used to assess survival.
In vivo studies in CD-1 nude mice bearing D341 medulloblastoma tumors
D341 cells (2.0 × 104 cells per mouse) were injected into the cerebellum of CD-1 nude mice as described above. Six days after implantation of tumor cells, the animals were injected i.t with 5 × 106 PFUs of vMyxgfp or DV every other day for a total of three injections. For combination therapy, animals were randomly divided into four groups (eight animals per group): (a) DV controls, (b) treatment with vMyxgfp alone, (c) treatment with rapamycin alone, and (d) treatment with vMyxgfp in combination with rapamycin. Virus was administered with 5 mg/kg/d rapamycin injected i.p. beginning 5 days after tumor implantation (five times weekly for 2 weeks). For survival studies (five animals per group), animals were followed until sacrifice was required or the experiment was terminated. The brain, spinal cord, and major organs were removed for histological examination. For studies of myxoma virus distribution, animals were sacrificed 48 h after treatment (three animals per treatment group). The brain was imaged in situ using a stereotactic microscope with a GFP filter. To quantitate GFP expression within tumors, the entire cerebellum was cut into sagittal sections, and GFP expression in each section was imaged using a fluorescence stereomicroscope. The section with the largest amount of GFP expression was analyzed with ImagePro software to quantify both the area of GFP expression and the total tumor area. Animals were perfused with PBS, and the brains and spinal cords were removed, embedded with ornithine carbamyl transferase, and sectioned for immunohistochemistry.
Statistical analyses
Statistical analysis software (SAS Institute, Inc.) and GraphPad Prism (version 4; GraphPad Software, Inc.) were used for statistical analyses. Survival curves were generated using the Kaplan-Meier method. The log-rank test and two-way ANOVA were used to compare the distributions of survival times and tumor sizes, respectively. Four treatment groups (DV, rapamycin alone, vMyxgfp + rapamycin, and vMyxgfp alone) were used to investigate individual and combined effects on killing of medulloblastoma cells. We compared the effect of each combined regimen with each of the two individual treatment strategies as well as the control samples by means of a Student’s t test. All reported P values were two sided and considered to be statistically significant at P < 0.05.
Results
Myxoma virus productively infects and kills most medulloblastoma cell lines in vitro
Ten medulloblastoma cell lines were tested for susceptibility to infection and killing by myxoma virus by analyzing cytopathic effect, the expression of early and late viral genes, and cell viability. All medulloblastoma cell lines with the exception of the D384 cells were permissive to infection, as shown by evidence of visible cytopathic effect and the expression of both early (GFP) and late (β-galactosidase) viral markers 48 h after infection with vMyxgfp or vMyxlac, respectively (representative examples in Fig. 1A; data for D283, D425, TE671, and UW228 cells are not shown). The D384 medulloblastoma cell line and the mouse fibroblast cell line NIH3T3 were poorly permissive to infection with myxoma virus and expressed very low levels of virally encoded GFP and β-galactosidase (Fig. 1A). There was no evidence of cytopathic effect or viral gene expression in untreated or DV-treated cells at any time point assessed (data not shown).
Figure 1.
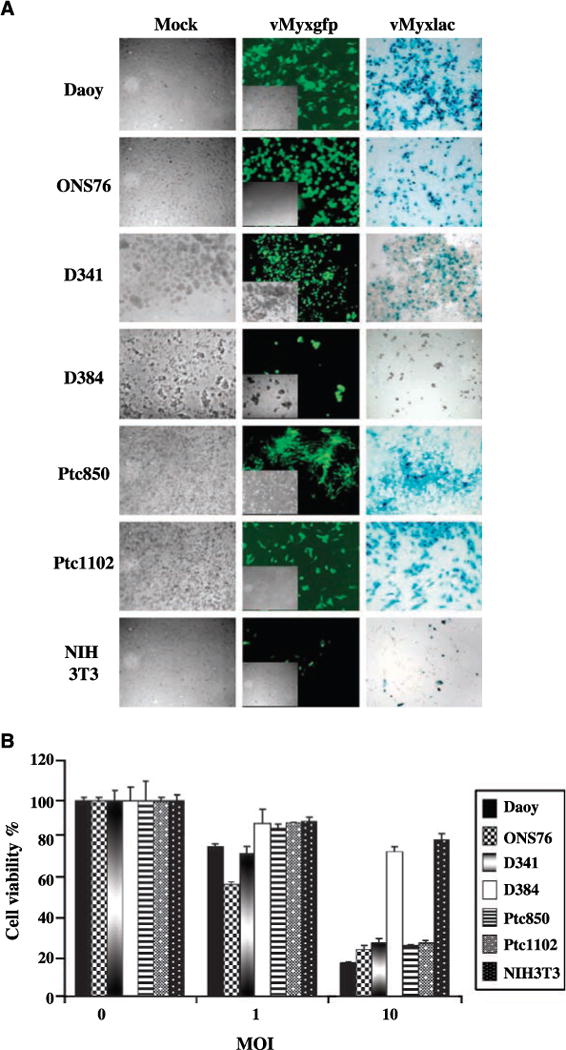
Effects of myxoma virus on established medulloblastoma cell lines in vitro. A, medulloblastoma (Daoy, ONS76, D341, D384, Ptc850, and Ptc1102) and murine fibroblast (NIH3T3) cell lines were infected with vMyxgfp or vMyxlac at a MOI of 5 and photographed 48h after infection. Middle, early viral gene expression (GFP) visualized by fluorescence microscopy. Inset, phase-contrast view of the same field. Right, late viral gene expression (β-galactosidase, blue foci) detected by X-gal staining. Magnification, ×100. Left, DV control. B, WST-1 assay comparing the effects of vMyxgfp on cell viability.
Cell viability assays (WST-1) confirmed the results from the cytopathic effect assays. Nine of the 10 medulloblastoma cell lines were susceptible to killing by myxoma virus and extensive cell death was observed 72 h after infection with 10 MOI of vMyxgfp (<30% of cells still viable; Fig. 1B). In contrast, D384 medulloblastoma cells and untransformed NIH3T3 cells were resistant to myxoma virus infection, with >80% of cells surviving 72 h after exposure to 10 MOI of vMyxgfp. Similar results were obtained when cell viability was assessed 72 h after infection with 10 MOI of vMyxlac (data not shown).
One of the benefits of replication-competent oncolytic viral therapy is the ability of viral progeny produced during the infection to spread and infect other cells within the tumor. To confirm the presence of a productive viral infection, expression of early (M-T7) and late (Serp-1) viral proteins was assessed by Western blotting 48 h after infection with 5 MOI of vMyxgfp. All of the six medulloblastoma cell lines susceptible to infection expressed both M-T7 and Serp-1 48 h after infection (Fig. 2A). The resistant D384 medulloblastoma cells expressed low levels of M-T7 but no Serp-1, whereas no M-T7 or Serp-1 protein could be detected in lysates from untransformed NIH3T3 cells.
Figure 2.
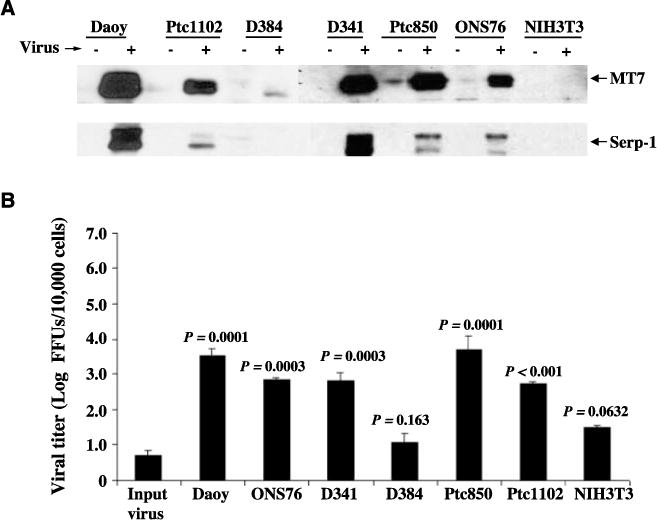
Myxoma virus productively infects medulloblastoma cell lines in vitro. A, Western blotting of whole-cell lysates (50 μg) for early (MT7) and late (Serp-1) myxoma virus gene expression following exposure to vMyxgfp (+) or no virus (−). B, viral titers were obtained in medulloblastoma and NIH3T3 cell lines after infection. Cells were infected with vMyxgfp (MOI of 0.1), incubated for 72 h, and lysed using three rounds of freeze-thawing to extract viral particles. The viral titers of samples were determined using a standard plaque titration assay on BGMK cells. Columns, mean focus forming units (FFU) of triplicate wells; bars, SD.
Myxoma virus titers in supernatants from susceptible medulloblastoma cell lines were increased ~3- to 5-fold compared with the original input viral titer (Fig. 2B) and cell-line specific differences in the extent of viral replication correlated with the susceptibility of a given cell line to viral infection. There was no significant viral replication in D384 or NIH3T3 cells.
Pretreatment with rapamycin promotes myxoma virus– mediated oncolysis and enhances viral replication in medulloblastoma cell lines in vitro
Treatment with rapamycin in vitro increases myxoma virus tropism in poorly permissive human cell lines (33). To determine whether pretreatment with rapamycin enhanced myxoma virus oncolysis of medulloblastoma cell lines in vitro, cell viability was assessed 72 h after infection in the presence or absence of rapamycin. Treatment with vMyxgfp and rapamycin resulted in greater cell killing than either treatment alone for Daoy, D341, and D384 cell lines (P < 0.001, t test, Fig. 3A and B; P < 0.05, t test, Fig. 3C). Combined treatment with rapamycin and myxoma virus had no effect on the viability of the control NIH3T3 cell line (P > 0.05, t test; Fig. 3D).
Figure 3.
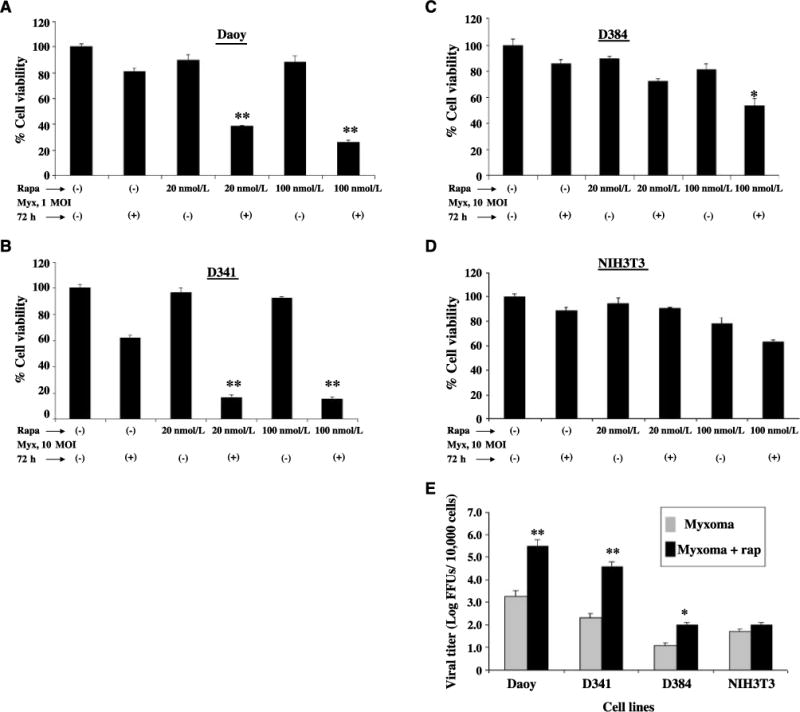
Effect of rapamycin combined with myxoma virus on medulloblastoma cell lines in vitro. A to D, the viability of medulloblastoma cell lines and murine fibroblast cell line (as a negative control) was measured using the WST-1 assay 72 h after infection with vMyxgfp in the presence (+) or absence (−) of rapamycin (Rapa). Myx, myxoma. E, cells were incubated for 1 h in medium alone or in medium containing 20 nmol/L rapamycin (rap) and then infected with 0.1 MOI of vMyxgfp as described in Materials and Methods. Viral titers were quantitated 72 h after infection using a standard plaque titration assay on BGMK cells. Columns, mean FFU of triplicate wells; bars, SD.
We then tested whether replication of myxoma virus was enhanced in cells treated with rapamycin. Treatment of medulloblastoma cell lines with both vMyxgfp and rapamycin increased viral titers 72 h after infection compared with virus alone (Fig. 3E). Daoy and D341 cells, which were fully susceptible to myxoma virus infection, showed significant increases in viral titers in the presence of rapamycin. There was a very small increase in viral titer in D384 cells treated with rapamycin, but the level of virus replication was still less than that observed in either Daoy or D341 cells in the absence of rapamycin. The mTOR inhibitor had no significant effect on viral titers in vMyxgfp-treated NIH3T3 cells.
Rapamycin increases activation of Akt in medulloblastoma cells
The PI3K/Akt pathway is constitutively activated in many medulloblastoma cells following stimulation of the IGF-I receptor (21, 39, 40), and susceptibility of human cancer cells to infection with myxoma virus is directly correlated to levels of activated Akt (18, 33). Rapamycin increases both Akt activation and myxoma virus replication in a variety of different cancer cell lines in vitro (33), but its effect on medulloblastoma cells had not been evaluated. To determine whether similar effects were observed when medulloblastoma cells were treated with rapamycin, Daoy, D341, D384, and NIH 3T3 cells were infected with DV or vMyxgfp (MOI of 5), with or without rapamycin pretreatment. Forty-eight hours after infection, levels of phosphorylated (and activated) Akt and p70S6K (a downstream substrate and effector of mTOR) were determined by Western blotting. As shown in Fig. 4A and B, rapamycin alone and vMyxgfp alone were able to increase phosphorylation of both Ser473 and Thr308 of Akt in Daoy and D341 cells (Fig. 4A and B, lanes 2 and 3). UV-inactivated vMyxgfp virus had no effect on phosphorylation levels of Akt (data not shown). Combined treatment with rapamycin and vMyxgfp also increased levels of phosphorylated Akt compared with untreated control cells, with the extent of activation being similar to that seen in cells treated with rapamycin alone (Fig. 4A and B, compare lanes 2 and 4). In both Daoy and D341 cells, levels of phosphorylated p70S6K were unchanged by infection with vMyxgfp alone (Fig. 4A and B, compare lanes 1 and 3), but, as expected, no phosphorylated p70S6K could be detected in cells treated with rapamycin, indicating that rapamycin was effectively inhibiting mTOR under the conditions used (Fig. 4A and B, compare lanes 1 and 2). In the poorly permissive D384 medulloblastoma cells, treatment with rapamycin or MV alone or in combination did not increase phosphorylation of Akt at either Ser473 or Thr308 in D384 cells (Supplementary Fig. S1A), and only a small increase in phosphorylation of Akt at Thr308 was seen when MV and rapamycin were used in combination in NIH3T3 cells, but not at Ser473, which is required for full activation (Supplementary Fig. S1B). These data are consistent with previous experiments where ectopic expression of constitutively active Akt1 render nonpermissive cancer cells susceptible to myxoma virus infection (18). Taken together, these results show that rapamycin not only inhibits mTOR kinase activity but also enhances Akt activation in human medulloblastoma cells that are susceptible to myxoma virus oncolysis.
Figure 4.
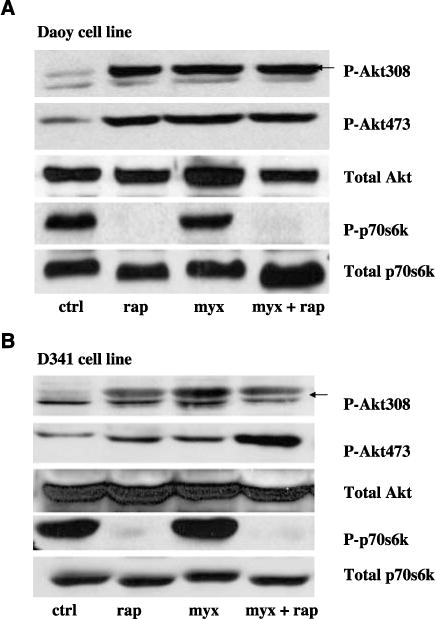
Rapamycin increases activation of Akt in human susceptible medulloblastoma cells. Daoy (A) and D341 (B) cells were pretreated with rapamycin (20 nmol/L) for 1 h, and then the cells were infected with vMyxgfp (5 MOI). Levels of phosphorylated p70S6K (P-p70S6K) and phosphorylated Akt (at Ser473 and Thr308; P-Akt473 and P-Akt308) in cell lysates were determined by Western blotting. Total Akt and mTOR levels were not changed.
I.t. administration of myxoma virus prolongs survival in Daoy tumor-bearing mice
To determine the efficacy of myxoma virus oncolysis in vivo, the effect of myxoma virus treatment in the presence or absence of rapamycin was evaluated in a Daoy medulloblastoma orthotopic xenograft model. Daoy cells were injected into the cerebellum of CD-1 nude mice to establish tumors. Twelve days later, vMyxgfp was injected i.t. (5 × 106 PFUs per mouse) and animals were monitored to assess survival. A single i.t. injection of vMyxgfp prolonged survival (P = 0.0021; Fig. 5A) and produced long-term survivors [three of five (60%) mice] that were apparently cured. The median survival of live virus (LV)-treated animals was not reached because only two of the five animals treated died during the course of the experiment (which lasted a total of 223 days). In contrast, all DV-treated animals [five of five (100%) mice] died with a significantly shorter median survival of 75 days (P = 0.0021, log-rank test). This survival experiment was repeated with similar results (data not shown). Histologic analysis showed that all of the DV-treated mice had large tumors in the cerebellum with compression of the brain stem (Fig. 5B). The two LV-treated animals that died both had recurrent or residual tumor in the brain stem (data not shown). Two LV-treated long-term survivors had no residual tumor either in the cerebellum or elsewhere in the brain (data not shown), whereas one LV-treated long-term survivor had a very tiny residual tumor (Fig. 5B).
Figure 5.
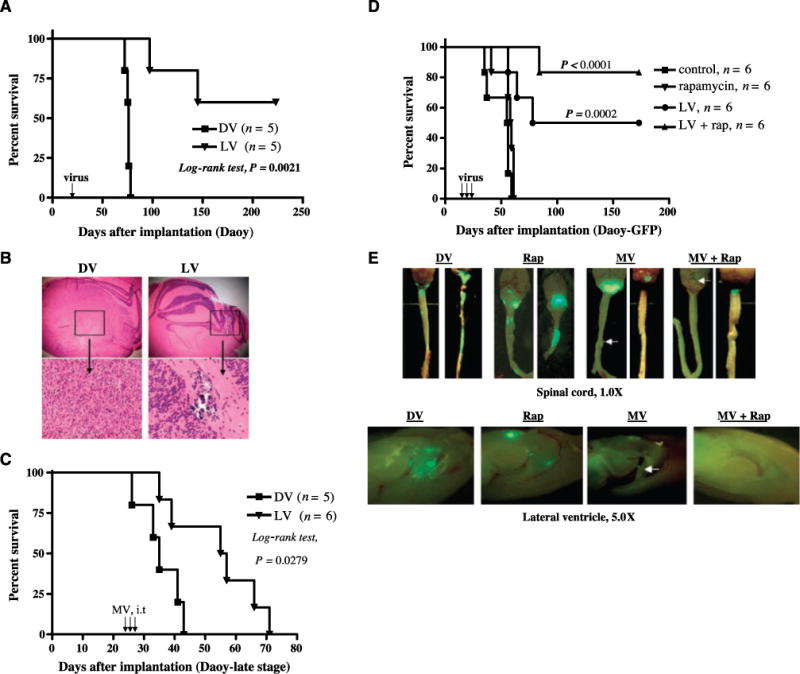
Effects of i.t. injection of myxoma virus in the presence or absence of rapamycin on survival and metastasis in Daoy tumor-bearing CD-1 nude mice. A, Kaplan-Meier survival analysis of animals implanted in the cerebellum with Daoy (1 × 105 cells per mouse) cells and treated with either DV or vMyxgfp (5 × 106 PFUs per mouse, single injection). The LV-treated animals (n = 5) survived significantly longer than DV-treated controls (n = 5; P = 0.0021, log-rank test). B, representative histologic analysis in a live vMyxgfp- and a DV-treated mouse. Magnifications, ×25 (top) and ×100 (bottom). Left, H&E of the DV-treated mouse brain tumor; right, H&E of the vMyxgfp-treated mouse brain. C, Kaplan-Meier survival analysis of mice implanted with Daoy (2 × 105 cells per mouse) and treated with either DV (n = 5) or vMyxgfp (n = 6; 5 × 106 PFUs per mouse, three injections) at “late stage” (25 d after tumor implantation). All P values are two sided. Virus injections (arrows in A and C). D, Kaplan-Meier plot showing the survival of Daoy-GFP-bearing mice after treatment with DV (n = 6), vMyxgfp (n = 6), rapamycin (n = 6), or a combination of both vMyxgfp and rapamycin (n = 6). E, representative spinal cords and ventricle metastases (green fluorescence indicates GFP-expressing tumor cells) in animals treated with DV (n = 3), rapamycin (n = 3), vMyxgfp (n = 3), and vMyxgfp plus rapamycin (n = 3). Arrows, spinal cord or ventricle metastasis. Magnifications, ×10 (top) and ×50 (bottom).
To better model the clinical situation of a large established tumor, we used a “late-stage” animal model to test the efficacy of vMyxgfp (Fig. 5C). For these experiments, vMyxgfp was injected i.t. 25 days after tumor implantation (54 × 106 PFUs per mouse) on 3 separate days. Multiple injections of vMyxgfp significantly prolonged survival of “late-stage” animals bearing Daoy medulloblastoma tumors. The median survival of DV-treated animals was 35 days, whereas LV-treated animals had a median survival of 56 days (P = 0.0279, log-rank test; Fig. 5C).
Rapamycin enhances myxoma virus oncolysis and reduces metastasis in vivo in Daoy-GFP tumor-bearing mice
Because rapamycin enhanced myxoma virus killing of medulloblastoma cell lines in vitro, we tested combination therapy with myxoma virus and rapamycin in the Daoy-GFP medulloblastoma mouse model to see if rapamycin could also enhance myxoma virus oncolysis in vivo. Daoy-GFP tumor-bearing mice were created by injecting 2 × 105 cells into the cerebellum of CD-1 nude mice. Eleven days after tumor implantation, rapamycin or a vehicle control was injected i.p. The following day, DV or vMyxgfp was injected i.t. Treatment with live vMyxgfp alone significantly enhanced survival of mice harboring Daoy-GFP human medulloblastoma xenografts (P = 0.0002 compared with DV control; Fig. 5D), but treating the mice with a combination of vMyxgfp and rapamycin was even more effective (P < 0.0001 compared with DV control; Fig. 5D). Treatment with a combination of vMyxgfp and rapamycin also significantly prolonged survival compared with treatment with vMyxgfp alone (P = 0.0398; Fig. 5D). Rapamycin alone had a small effect on survival but this difference was not statistically significant (P = 0.1167 compared with DV control; Fig. 5D). Three animals in each group were sacrificed 30 days after virus treatment and ventricular and spinal cord metastases were quantified by counting GFP-expressing tumor foci (Fig. 5E). We found that all DV (n = 3) or rapamycin-treated (n = 3) animals had at least five to six spinal cord metastases per mouse and at least two to three ventricular metastases per mouse. For animals treated with myxoma virus alone (n = 3), one animal had two spinal cord metastases and one animal had a single ventricular metastasis. In animals treated with a combination of myxoma virus and rapamycin (n = 3), no ventricular or spinal cord metastases were observed.
I.t. administration of myxoma virus prolongs survival in reovirus-resistant D341 tumor-bearing mice
The D341 medulloblastoma cell line is resistant to oncolysis by reovirus (37); however, our in vitro experiments showed that these cells are susceptible to infection and killing by myxoma virus. To analyze the in vivo efficacy of myxoma virus against medulloblastoma tumors that are resistant to other oncolytic agents, we determined whether i.t. injection of myxoma virus could induce tumor regression in CD-1 nude mice bearing orthotopic D341 tumors. Six days after tumor implantation in the cerebellum, mice were given three i.t. injections of vMyxgfp (5 × 106 PFUs per mouse) every other day. Treatment with live vMyxgfp significantly prolonged survival (median survival of LV-treated animals was 20.5 days versus 13 days for DV-treated animals; P = 0.0008, log-rank test; Fig. 6A). Histologic analysis showed that all of the DV-treated mice and LV-treated mice had large tumors in the cerebellum. The D341 tumor model showed more invasion into the brain and increased compression of the brain stem compared with the Daoy tumor model (Fig. 6B). Immunohistochemical analysis showed expression of the viral protein MT7 in tumors from vMyxgfp-treated animals, indicating the presence of a productive viral infection in vivo (Fig. 6B).
Figure 6.
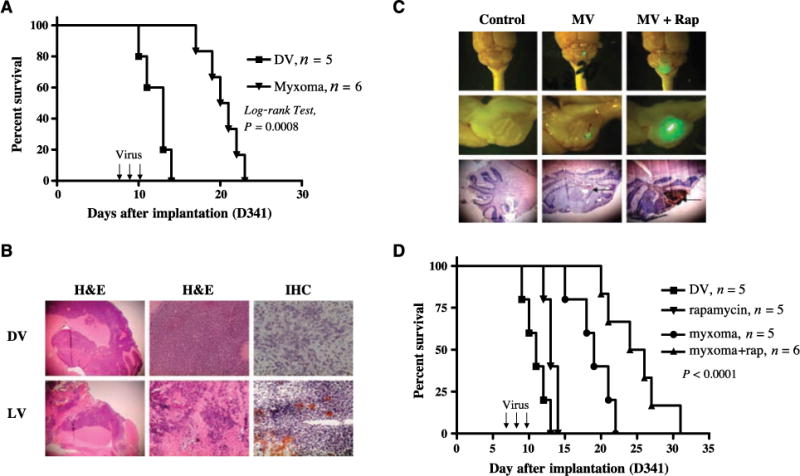
Administration of myxoma virus inhibits growth of reovirus-resistant D341 medulloblastoma tumors in vivo and combined therapy with rapamycin synergistically increases myxoma virus oncolysis in D341 tumor-bearing mice. A, Kaplan-Meier survival curves of nude mice harboring human D341 tumor treated with DV (n = 5) or vMyxgfp (n = 6; 5 × 106 PFUs per mouse). Arrows, day of virus administration. B, representative histologic analysis in a vMyxgfp- and a DV-treated mouse. Magnifications, ×25 (left), ×100 (middle), and ×400 (right). Top left and middle, H&E staining of the DV-treated mouse brain tumor; bottom left and middle, H&E staining of the LV-treated mouse brain. Right, immunohistochemistry (IHC) staining for myxoma virus protein. C, histologic characterization and GFP virus distribution of i.t. administered vMyxgfp in mice bearing D341 tumor. Mice bearing D341 tumor were treated i.p. with rapamycin 5 d after tumor cell implantation. The next day, animals were treated with vMyxgfp (i.t.) at a dose of 5 × 106 PFUs per mouse. Animals were sacrificed 48h after virus infection. Top and middle, photomicrograph of GFP-labeled virus in cerebellum tumor (n = 3 mice per group. Magnification, top, ×10; middle, ×25. Arrows, GFP virus expression. Bottom, immunohistochemical staining for myxoma virus protein M-T7. Arrow, brown staining. Magnification, ×25. D, Kaplan-Meier plot showing the survival of D341 tumor-bearing mice after treatment with DV (n = 5), vMyxgfp (n = 5), rapamycin (n = 5), or a combination of both vMyxgfp and rapamycin (n = 6). All P values are two sided.
Combination therapy with myxoma virus and rapamycin enhances viral replication and survival of reovirus-resistant D341 medulloblastoma tumor-bearing mice
To determine if pretreatment with rapamycin enhanced myxoma virus replication in D341 tumors in vivo, we implanted D341 cells into the cerebellum of CD-1 nude mice and treated the mice with vMyxgfp and rapamycin using the same doses and schedule of injections described for the Daoy tumor-bearing mice (early-stage model). Animals were sacrificed 48 h after virus injection and the brains were examined using fluorescence stereomicroscopy. Treatment with rapamycin 24 h before injection of myxoma virus markedly increased the anatomic area of viral GFP expression (~56% of tumor cells expressed GFP; Fig. 6C) compared with treatment with vMyxgfp alone (~7.0% of tumor cells expressed GFP; Fig. 6C). Immunohistochemistry confirmed that the increase in GFP expression corresponded to an increase in expression of the M-T7 viral protein within tumors (Fig. 6C).
To determine if combination therapy resulted in a significant prolongation of survival, we implanted D341 cells into the cerebellum of CD-1 nude mice and 6 days later treated with DV, vMyxgfp, rapamycin alone, or vMyxgfp and rapamycin using the doses and injection schedules described above. Treatment of animals with vMyxgfp significantly prolonged survival compared with DV-treated animals (P = 0.0018); however, mice treated with rapamycin and i.t. injections of vMyxgfp (median survival of 25 days) survived significantly longer than animals treated with vMyxgfp alone, rapamycin alone, or DV (median survival of 19, 13, and 11 days, respectively; P < 0.0001, log-rank test, combination group compared with control group; Fig. 6D).
Discussion
Although there are ongoing clinical trials evaluating oncolytic virotherapy of malignant brain tumors in adults (8–10), there are little data available on the toxicity and efficacy of such an approach in children with medulloblastoma (11, 37). This is the first demonstration of the potential usefulness of replication-competent myxoma virus against experimental models of medulloblastoma, both as a single agent and in combination with rapamycin. Myxoma virus infects and kills most medulloblastoma tumor cell lines tested in vitro, prolongs survival of animals bearing implanted medulloblastoma tumors, is safe when administered i.c. in normal mice (41), and is nonpathogenic in humans (13). Myxoma virus in combination with rapamycin dramatically reduced spinal cord and ventricular metastases in a medulloblastoma mouse model. However, myxoma virus and reovirus were unable to induce regression of uninoculated tumors in bilateral mouse glioma models (7, 12). Insufficient viral delivery is a key limitation in the treatment of intraparenchymal multifocal malignant gliomas (see ref. 41 and references therein), and it is likely that the successful targeting of metastatic cells in medulloblastoma is due to more efficient viral spread in the cerebral spinal fluid, where medulloblastoma cells ordinarily disseminate. This observation may be important therapeutically because decreased metastasis could allow the use of lower doses of radiation to a much smaller area of the central nervous system and significantly reduce treatment-related toxicity in children with developing nervous systems.
Although reovirus is effective in prolonging survival and reducing metastasis in medulloblastoma animal models (37), it is also clear that some brain tumor cells are resistant to reovirus oncolysis (37, 42). The ability of myxoma virus to productively infect and kill reovirus-resistant D341 cells both in vitro and in vivo suggests that myxoma virus may be a valuable therapeutic option in reovirus-resistant tumors and may address the problem of innate or acquired resistance. Differences in the susceptibility of particular tumor cell types to distinct oncolytic viruses probably reflect the differing tropism requirements of a given virus combined with the heterogeneity that is observed in the signaling microenvironments of different tumors. Indeed, whereas myxoma virus requires Akt activation for permissivity, susceptibility to reovirus oncolysis is highly correlated with the presence of activated Ras (37, 42) and high levels of protease activity in the tumor milieu (43). The unique pattern of up-regulation or down-regulation of specific signaling pathways will therefore determine the success or failure of viral oncolysis of a given tumor. Combination therapies with agents that selectively modify important signaling pathways have the potential to significantly increase the efficacy of oncolytic viruses.
We found that combination therapy with myxoma virus and the mTOR inhibitor rapamycin was significantly more effective than either agent alone in killing medulloblastoma cells both in vitro and in vivo. This result is consistent with our previous in vitro demonstration of the synergy between rapamycin and myxoma virus oncolysis in nonmedulloblastoma cell lines (33) and is the first demonstration that a similar synergy occurs in vivo. Rapamycin has several additional properties that make it an attractive candidate for combination therapy with myxoma virus. Rapamycin has no known inhibitory effect on viral replication in medulloblastoma (data not shown) and thus it does not interfere with virally mediated oncolysis. The lipophilic nature of rapamycin enables it to easily cross the blood-brain barrier. Rapamycin has low toxicity and can directly inhibit brain tumor growth by blocking tumor cell proliferation and angiogenesis (25–30), in addition to its enhancement of myxoma virus tropism. Because it has already been used in the clinic (23), rapamycin is immediately available for use in clinical trials designed to evaluate its efficacy in combination with myxoma virus or other oncolytic viruses of interest. The successful use of rapamycin to specifically modulate a signaling pathway known to be important in myxoma virus tropism suggests that a rationally targeted therapeutic strategy combining mTOR inhibitors and myxoma virus could enhance viral oncolysis in patients.
The mechanism by which rapamycin enhances myxoma virus oncolysis of human medulloblastoma is not known. It may be due to inhibition of the innate immune response, disruption of the IFN response, the induction of apoptosis or autophagy, and/or alterations in other signaling pathways (23, 27, 44). Rapamycin is also an immunosuppressant, and this may enhance its effectiveness in vivo but not in vitro. We found, as described previously in other types of cancer cell lines (33), that rapamycin-mediated sensitization of medulloblastoma cells to myxoma virus infection correlated with an increase in the level of Akt activation. There is substantial evidence that the IGF-I signaling pathway is highly activated in medulloblastoma and contributes to transformation (23, 40), and it is possible that rapamycin up-regulates Akt in medulloblastoma cells by affecting IGF-I receptor signaling. Studies of insulin and IGF-I signaling in Drosophila (45) and in mammalian cells (46, 47) describe a negative feedback loop in which mTOR inhibits insulin receptor substrate-1 (IRS-1)–mediated signaling by activating p70S6K, which subsequently phosphorylates IRS-1 and promotes its degradation via the proteasome. This leads to down-regulation of the PI3K/Akt pathway, which is normally activated downstream of insulin or IGF-I following recruitment of PI3K to active receptor/IRS-1 signaling complexes. A similar negative feedback loop operating through mTOR may exist in medulloblastoma cells. Alternatively, Akt-independent signaling effects downstream of mTOR may be involved in the synergistic interaction of rapamycin and myxoma virus. The molecular mechanisms through which rapamycin is able to enhance myxoma virotherapy are currently under investigation in our laboratories.
Our study has several limitations. First, we have not yet evaluated myxoma virus in immunocompetent models of medulloblastoma where distribution of the virus within the brain may be impeded by significant immune responses. Second, the precise mechanisms by which myxoma virus kills medulloblastoma cells and the mechanisms through which rapamycin enhances oncolysis by myxoma virus are not yet fully understood. It will be of particular importance to determine whether Shh signaling is important in the mechanism of action of myxoma virus and rapamycin because it has been shown recently that the Shh pathway is irreversibly suppressed in medulloblastoma cells that are cultured in vitro (48), although there is strong evidence that Shh signaling is important in medulloblastoma tumorigenesis in vivo (22, 23). This finding raises concerns about the use of cultured medulloblastoma tumor cells in preclinical animal models that test therapies that may be affected by Shh signaling. We are now exploring strategies that will enable us to pursue preclinical studies in more appropriate immunocompetent animal models to increase our understanding of the mechanisms involved in viral oncolysis of medulloblastoma.
Supplementary Material
Acknowledgments
Grant support: Kids Cancer Care Foundation (P.A. Forsyth and D.L. Senger), a Program Project grant from National Cancer Institute of Canada with funds from the Terry Fox Foundation (P.A. Forsyth, D.L. Senger, and G. McFadden), and the Clark H. Smith Integrative Brain Tumour Research Centre (P.A. Forsyth). T. Alain is funded by the Alberta Heritage Foundation for Medical Research and is the recipient of a Fellowship from the Canadian Institutes of Health Research. M.M. Stanford is the recipient of a Postdoctoral Fellowship provided by the Pamela Greenaway Kohlmeier Translational Breast Cancer Research Unit of the London Regional Cancer Program. G. McFadden is an International Scholar of the Howard Hughes Medical Institute.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Walker DA, Wilne S. Treatment of medulloblastoma in young children. Lancet Oncol. 2005;6:541–2. doi: 10.1016/S1470-2045(05)70259-X. [DOI] [PubMed] [Google Scholar]
- 2.Ribi K, Relly C, Landolt MA, et al. Outcome of medulloblastoma in children: long-term complications and quality of life. Neuropediatrics. 2005;36:357–65. doi: 10.1055/s-2005-872880. [DOI] [PubMed] [Google Scholar]
- 3.Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 2004;5:209–18. doi: 10.1016/S1470-2045(04)01424-X. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Pomeroy SL, Cairncross JG. Focus on central nervous system neoplasia. Cancer Cell. 2002;1:125–8. doi: 10.1016/s1535-6108(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 5.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–76. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, Conrad C, Fueyo J, Gomez-Manzano C, Liu TJ. Oncolytic adenoviruses for malignant glioma therapy. Front Biosci. 2003;8:d577–88. doi: 10.2741/923. [DOI] [PubMed] [Google Scholar]
- 7.Lun X, Yang W, Alain T, et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–90. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65:279–89. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 9.Colombo F, Barzon L, Franchin E, et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12:835–48. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- 10.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 11.Stolarek R, Gomez-Manzano C, Jiang H, et al. Robust infectivity and replication of Delta-24 adenovirus induce cell death in human medulloblastoma. Cancer Gene Ther. 2004;11:713–20. doi: 10.1038/sj.cgt.7700731. [DOI] [PubMed] [Google Scholar]
- 12.Yang WQ, Lun X, Palmer CA, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–76. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 13.McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005;3:201–13. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sypula J, Wang F, Ma Y, Bell J, McFadden G. Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol. 2004;8:103–14. [Google Scholar]
- 15.Cameron C, Hota-Mitchell S, Chen L, et al. The complete DNA sequence of myxoma virus. Virology. 1999;264:298–318. doi: 10.1006/viro.1999.0001. [DOI] [PubMed] [Google Scholar]
- 16.Shisler JL, Moss B. Immunology 102 at poxvirus U: avoiding apoptosis. Semin Immunol. 2001;13:67–72. doi: 10.1006/smim.2000.0297. [DOI] [PubMed] [Google Scholar]
- 17.Kerr P, McFadden G. Immune responses to myxoma virus. Viral Immunol. 2002;15:229–46. doi: 10.1089/08828240260066198. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Barrett JW, Stanford M, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci U S A. 2006;103:4640–5. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werden SJ, Barrett JW, Wang G, Stanford MM, McFadden G. M-T5, the ankyrin repeat, host range protein of myxoma virus, activates Akt and can be functionally replaced by cellular PIKE-A. J Virol. 2007;81:2340–8. doi: 10.1128/JVI.01310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raffel C. Medulloblastoma: molecular genetics and animal models. Neoplasia. 2004;6:310–22. doi: 10.1593/neo.03454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann W, Digon-Sontgerath B, Koch A, et al. Phosphatidylinositol 3′-kinase/AKT signaling is activated in medulloblastoma cell proliferation and is associated with reduced expression of PTEN. Clin Cancer Res. 2006;12:3019–27. doi: 10.1158/1078-0432.CCR-05-2187. [DOI] [PubMed] [Google Scholar]
- 22.Rao G, Pedone CA, Valle LD, et al. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23:6156–62. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- 23.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 2: PI3K/Akt/PTEN, mTOR, SHH/PTCH, and angiogenesis. Expert Rev Anticancer Ther. 2004;4:105–28. doi: 10.1586/14737140.4.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 25.Houchens HP, Ovejera AA, RIblet SM, Slagel DE. Human brain tumor xenografts in nude mice as a chemotherapy model. Eur J Cancer Clin Oncol. 1983;19:799–805. doi: 10.1016/0277-5379(83)90012-3. [DOI] [PubMed] [Google Scholar]
- 26.Geoerger B, Kerr K, Tang CB, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61:1527–32. [PubMed] [Google Scholar]
- 27.Takeuchi H, Kondo Y, Fujiwara K, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 28.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 30.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–61. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 31.Cinatl J, Jr, Cinatl J, Michaelis M, et al. Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res. 2003;63:1508–14. [PubMed] [Google Scholar]
- 32.Bennett JJ, Adusumilli P, Petrowsky H, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma134.5 deleted oncolytic herpes virus (G207) FASEB J. 2004;18:1001–3. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- 33.Stanford MM, Barrett JW, Nazarian SH, Werden S, McFadden G. Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. J Virol. 2007;81:1251–60. doi: 10.1128/JVI.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wetmore C, Eberhart DE, Curran T. The normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched. Cancer Res. 2000;60:2239–46. [PubMed] [Google Scholar]
- 35.Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–6. [PubMed] [Google Scholar]
- 36.Opgenorth A, Graham K, Nation N, Strayer D, McFadden G. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L and myxoma growth factor. J Virol. 1992;66:4720–31. doi: 10.1128/jvi.66.8.4720-4731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang WQ, Senger D, Muzik H, et al. Reovirus prolongs survival and reduces the frequency of spinal and leptomeningeal metastases from medulloblastoma. Cancer Res. 2003;63:3162–72. [PubMed] [Google Scholar]
- 38.Johnston JB, Nazarian SH, Natale R, McFadden G. Myxoma virus infection of primary human fibroblasts varies with cellular age and is regulated by host interferon responses. Virology. 2005;332:235–48. doi: 10.1016/j.virol.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 39.Wang JY, Del Valle L, Gordon J, et al. Activation of the IGF-IR system contributes to malignant growth of human and mouse medulloblastomas. Oncogene. 2001;20:3857–68. doi: 10.1038/sj.onc.1204532. [DOI] [PubMed] [Google Scholar]
- 40.Reiss K. Insulin-like growth factor-I receptor—a potential therapeutic target in medulloblastomas. Expert Opin Ther Targets. 2002;6:539–44. doi: 10.1517/14728222.6.5.539. [DOI] [PubMed] [Google Scholar]
- 41.Lun X, Senger DL, Alain T, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98:1546–57. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 42.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–12. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 43.Alain T, Kim M, Johnston RN, et al. The oncolytic effect in vivo of reovirus on tumour cells that have survived reovirus cell killing in vitro. Br J Cancer. 2006;95:1020–7. doi: 10.1038/sj.bjc.6603363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dilling MB, Dias P, Shapiro DN, et al. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer Res. 1994;54:903–7. [PubMed] [Google Scholar]
- 45.Stocker H, Radimerski T, Schindelholz B, et al. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–65. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 46.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 47.Harrington LS, Findlay GM, Gray A, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasai K, Romer JT, Lee Y, et al. Shh pathway activity is down-regulated in cultured medulloblastoma cells: implications for preclinical studies. Cancer Res. 2006;66:4215–22. doi: 10.1158/0008-5472.CAN-05-4505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


