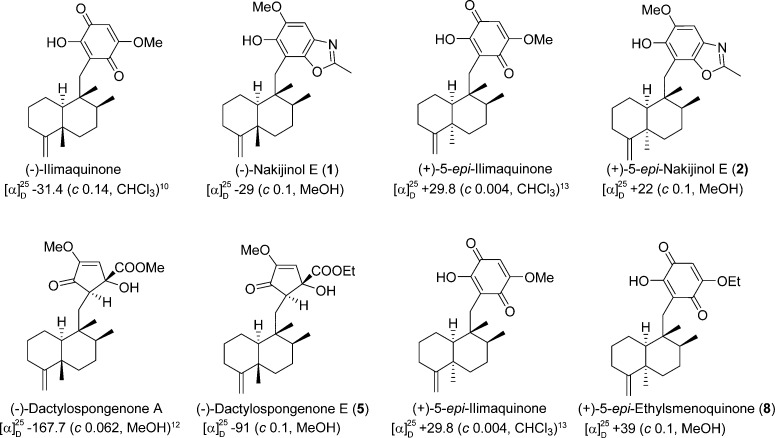
Figure 3.
Establishment of absolute configuration of optically pure compounds in this study (i.e., compounds 1, 2, 5, and 8). The trans-friedodrimanes (−)-nakijinol E (1) and (−)-dactylospongenone E (5) displayed negative specific rotations in accord with those of (−)-ilimaquinone8,25 and (−)-dactylospongenone A.17 Inversion of the C-5 absolute configuration with formation of the cis-friedodrimane core structure led to a significant conformational change that is exemplified by a consistent change in the sign of the optical rotation. Thus, the specific rotations of (+)-5-epi-nakijinol E (2) and (+)-5-epi-ethylsmenoquinone (8) are consistent with those of the analogous (+)-5-epi-ilimaquinone.15,26