Figure 4.
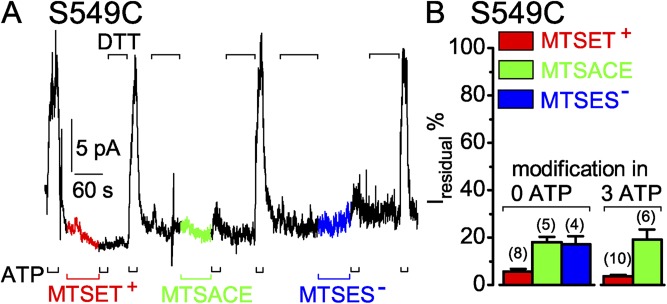
S549C CFTR channels are readily modified by MTS reagents when closed. (A) Immediately after 60-s applications of 5 µM MTSET+ (red trace and bar), 100 µM MTSACE (green trace and bar), or 100 µM MTSES− (blue trace and bar) to closed S549C channels in the absence of ATP, brief exposures to 3 mM ATP (black bars below record) assessed residual channel activity; the time constant of Ca2+-dependent Cl− current decay in this patch was 0.2 s. Exposures to 10 mM DTT (black bars above record) fully restored ATP-activated current by releasing adducts after each modification. (B) Relative amplitude of residual ATP-dependent current (Iresidual %) of modified S549C channels. For modification while channels were closed (left, 0 ATP), residual ATP-activated current (peak current a few seconds after adding ATP minus baseline current just before ATP addition) of modified channels was compared with the average of peak ATP-activated currents of the same channels after full recovery from modification, and just before modification by MTSET+ (red bar, 6 ± 1%; n = 8 measurements in six patches), by MTSACE (green bar, 18 ± 2%; n = 5 measurements in four patches), or by MTSES− (blue bar, 17 ± 3%; n = 4 measurements in three patches). For modification while channels were opening and closing in ATP (right, 3 mM ATP), residual ATP-dependent current (measured as the difference before and after ATP removal) of modified channels was compared with ATP-activated current of the same channels immediately before modification by ≥50 µM MTSET+ (red bar, 4 ± 1%; n = 10 measurements in five patches) or by ≥50 µM MTSACE (green bar, 18 ± 6%; n = 4 measurements in four patches). Error bars represent mean ± SEM.