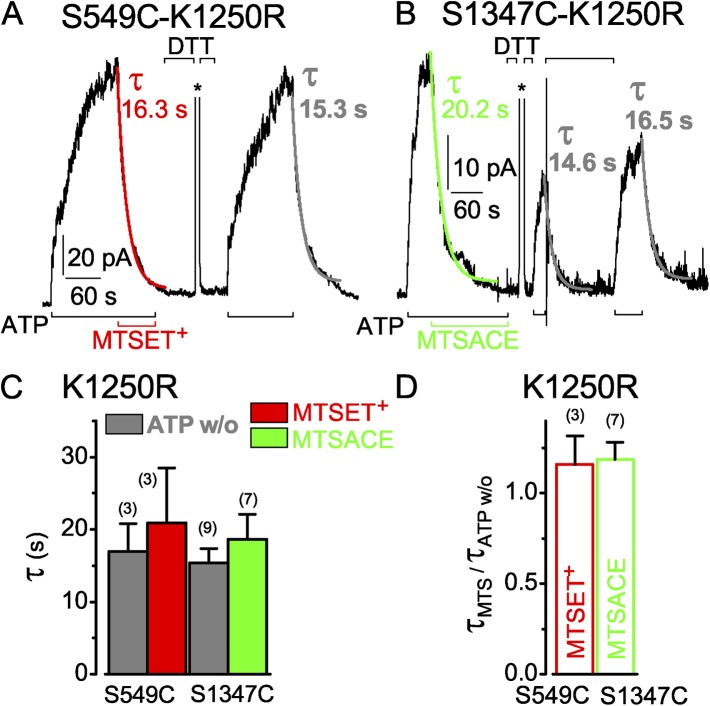
Figure 7.
Hydrolysis-impairing mutation, K1250R, of the conserved Walker A lysine in the active composite site similarly slows current decay after ATP washout and upon MTS modification of both S549C and S1347C channels. (A and B) ATP-activated (3 mM, black bars below records) currents of S549C-K1250R (A) and S1347C-K1250R (B) CFTR channels with single-exponential fits to current decline upon ATP removal (gray, τATP w/o) or modification (τMTS) by 50 µM MTSET+ (red) or MTSACE (green); 20 mM DTT (black bars above records) restored activation of currents by ATP; asterisks above the records mark brief activations of Ca2+-dependent Cl− currents to monitor speed of solution exchange (0.3 s in A and 0.2 s in B). (C) Average τATPw/o (gray bars) with corresponding average τMTS from the same patches (left, S549C-K1250R: gray bar, w/o, 17 ± 3.8 s; red bar, MTSET+, 20.9 ± 7.6 s; n = 3 measurements in three patches; right, S1347C-K1250R: gray bar, w/o, 15.4 ± 2.0 s; green bar, MTSACE, 18.6 ± 3.5 s; n = 9 and 7 measurements, respectively, in three patches). (D) Averages of individual ratios of washout and modification time constants determined for each pair of measurements (from experiments of C; red open bar, S549C-K1250R, τMTSET/τATPw/o, 1.2 ± 0.2; green open bar, S1347C-K1250R, τMTSACE/τATPw/o, 1.2 ± 0.1). Error bars represent mean ± SEM.