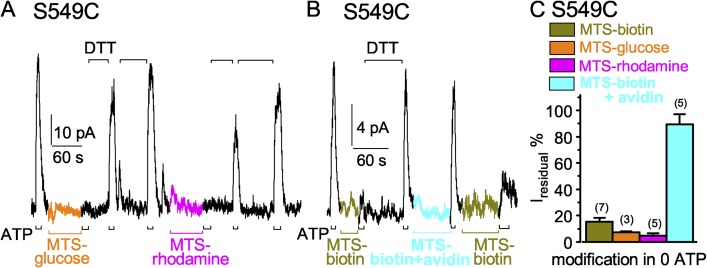
Figure 8.
Larger MTS reagents also readily modify S549C CFTR channels when they are closed. (A and B) ATP-activated current (3 mM, black bars below record) was strongly diminished after modification of closed S549C CFTR channels by ≤60-s exposures to larger MTS reagents, 20 µM MTS-glucose (A; orange bar), 5 µM MTS-rhodamine (A; magenta bar), 5 µM MTS-biotin (B; dark yellow bar), and MTS-biotin–avidin complex (B; 5 µM biotin plus 5 µM avidin, cyan bar), all in the absence of ATP; 10 mM DTT (black bars above records) released adducts after each modification; the time constants of Ca2+-dependent Cl− current decays in these patches were 0.5 s (A) and 0.2 s (B). (C) Amplitude of residual ATP-activated current (Iresidual %), relative to ATP-activated current before modification, for S549C channels modified, while closed (in 0 ATP), by MTS-biotin (dark yellow bar, 15 ± 3%, n = 7 measurements), by MTS-glucose (orange bar, 7 ± 1%, n = 3 measurements), by MTS-rhodamine (magenta bar, 5 ± 2%, n = 5 measurements), or by MTS-biotin–avidin complex (cyan bar, 90 ± 8%, n = 5 measurements). Error bars represent mean ± SEM.