Abstract
Objective
The purpose of this investigation was to understand the metabolic adaptations to a short-term (5 days), isocaloric, high fat diet (HFD) in healthy, young males.
Methods
Two studies were undertaken with 12 subjects. Study 1 investigated the effect of the HFD on skeletal muscle substrate metabolism and insulin sensitivity. Study 2 assessed the metabolic and transcriptional response in skeletal muscle to the transition from a fasted-to-fed state using a high fat meal challenge prior to and following 5 days of HFD.
Results
Study 1 showed no effect of a HFD on skeletal muscle metabolism or insulin sensitivity in fasting samples. Study 2 showed that a HFD elicits significant increases in fasting serum endotoxin, and disrupts the normal postprandial excursions of serum endotoxin, and metabolic and transcriptional responses in skeletal muscle. These effects following 5 days of HFD were accompanied by an altered fasting and postprandial response in the ratio of phosphorylated to total p38 protein. These changes all occurred in the absence of alterations in insulin sensitivity.
Conclusions
Our findings provide evidence for early biological adaptations to high fat feeding that proceed and possibly lead to insulin resistance.
Keywords: Human, high fat diet, skeletal muscle, metabolism
Introduction
High fat diet (HFD) induced obesity is associated with a modest elevation in circulating endotoxin concentrations (termed metabolic endotoxemia) and insulin resistance in rodents (1, 2, 3). We (4) have previously reported that lipopolysaccharide suppresses skeletal muscle homogenate fatty acid oxidation and increases glucose oxidation in rodents. However, whether a HFD increases circulating endotoxin and produces dysregulated skeletal muscle substrate metabolism in non-obese humans is unknown. Therefore, the purposes of this investigation were to determine if a short-term HFD elicited metabolic endotoxemia in non-obese humans, and adversely affected whole body insulin sensitivity and skeletal muscle substrate metabolism when transitioning from a fasting-to-fed state.
Methods and Procedures
Experimental design
Twelve college-aged (mean, 21 ± 1 year), nonobese (mean body mass index (BMI), 22.3 ± 3.9 kg/m2) males volunteered for the study. They were free from overt disease and not taking any medications. All were sedentary (<2 days/week for <20 min/day), non-smoking, and weight stable (± 2 kg) for the previous 6 months. The study protocol was approved by the Institutional Review Board at Virginia Tech (Blacksburg, VA).
Study 1
Subjects (n=6) consumed a lead-in control diet (55% carbohydrate, 15% protein, and 30% fat [11% SFA]) that was isocaloric to their habitual diet for one week prior to HFD. Subsequently, subjects were provided a HFD for 5 days. The composition of the HFD was 30% carbohydrate, 15% protein, and 55% fat (25% SFA) and designed to be isocaloric to the lead-in control diet. Subjects reported to our metabolic kitchen daily to eat breakfast, receive remaining meals for the day, and to have body weight measured. A skeletal muscle biopsy from the vastus lateralis muscle and a 3-hour intravenous glucose tolerance test (IVGTT) were performed in the post-absorptive state (10–12 hour fast) before and after HFD (study time line is provided in supplementary material).
Study 2
Using the identical feeding paradigm as in study 1, a separate group of subjects (n=6) consumed a high fat meal consisting of 880 kcal (63% fat [10% SFA], 25% carbohydrate, and 12% protein) before and following HFD. Muscle biopsies were collected prior to (10–12 hour fast) and at 4 hours following the high fat meal challenge. A study time line is provided in supplementary material.
General Procedures
Extended methods are provided in supplementary material. Body weight and height were measured on a digital scale and stadiometer, respectively. Body composition was determined by DEXA (Prodigy Advance, GE Healthcare, Madison, WI). Whole-body insulin sensitivity was assessed in study 1 using the IVGTT (5) (MINMOD Millennium Software) as previously described (6, 7) and by the Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) in both studies 1 and 2 (8). Serum endotoxin concentrations were determined using the PyroGene Recombinant Factor C endotoxin detection assay (Lonza, Basel, Switzerland).
Dietary assessment
Energy requirements were estimated based on height, weight, age, and activity level (9). A research dietitian instructed volunteers to accurately report food and beverage intake and reviewed all records with the participants for accuracy and sufficient detail. Food intake records were analyzed with the Nutritionist Pro Diet Analysis Software (Axxya Systems, Stafford, TX).
Skeletal muscle biopsies and homogenate preparation
Biopsies samples were taken with suction from the vastus lateralis muscle under local anesthesia (1% lidocaine) using a modified Bergström needle as described previously (7). Skeletal muscle homogenates were prepared and measures of glucose oxidation and enzyme activities [Phosphofructokinase (PFK), citrate synthase (CS), and β-hydroxyacyl CoA dehydrogenase (β-HAD)] were performed as previously described (4, 10).
mRNA extraction, qRT-PCR, and western blotting
Samples were placed in Trizol (Invitrogen, Carlsbad, CA), snap-frozen in liquid nitrogen, and subsequently stored at −80°C until analysis. RNA was extracted using an RNeasy Mini Kit (Qiagen) and DNase I treatment (Qiagen, Valencia, CA). qRT-PCR was performed using an ABI PRISM 7900HT Sequence Detection System instrument and TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Primers and 5# FAM-labeled TaqMan probes were purchased as pre-validated assays (ABI). Relative quantification of target genes was calculated using the ΔCT method (Applied Biosystems User Bulletin no. 2 P/N 4303859). All samples were run in triplicate and expressed as target gene mRNA/cyclophilin B mRNA in arbitrary units. Muscle used for western blotting was placed in mammalian lysis buffer (50mM Tris-HCL, 1mM EDTA, 150mM NaCL, 0.1% Sodium dodecyl sulfate, 0.5% sodium deoxycholate, 0.01% Igepal CA) with protease and phosphatase inhibitor cocktail (Thermo Scientific, Waltham, MA), snap-frozen in liquid nitrogen, and subsequently stored at −80°C until analysis. Proteins (30 μg) were separated using a 10% Criterion-Tris- HCl gel (Bio-Rad, Hercules, CA) and subsequently transferred to PVDF membrane (Bio-Rad, Hercules, CA). Blots were probed with primary antibodies against phospho-p38 and total-38 (Cell Signaling, Danvers, MA; 1:1000) followed by anti-rabbit or anti-mouse secondary antibodies (Jackson Immuno Research Laboratories, West Grove, PA; 1:10,000). Proteins were visualized using Super-Signal Chemiluminescent Substrate (Pierce, Rockville, Il) and a ChemiDoc XRS Imaging System (BioRad, Hercules, CA). p38 protein was normalized to GAPDH (Santa Cruz Biotechnology, Dallas, TX; 1:1000).
Statistical analysis
All statistics were run using Prism 6 (GraphPad Software Inc., San Diego, CA). A paired t-test was used to determine differences in the resulting means from assays performed in the fasted state before and following the HFD (Study 1). A two-way repeated measures analysis of variance followed by a Sidak’s multiple comparison test was used to compare changes in variables of interest in response to the meal challenge before and following the HFD (Study 2). All data are presented as means ± standard error of the mean (SEM), and considered significant at P≤0.05.
Results
Body weight and body fat percentage did not change with HFD in study 1 or 2 (P>0.05) (Table 1). Whole body insulin sensitivity did not change following HFD in study 1 or 2 (both P>0.05) (Table 1).
Table 1.
Subject characteristics before and after acute HFD
| Study #1 (n=6) | Study #2 (n=6) | |||
|---|---|---|---|---|
|
| ||||
| Variable | Pre | Post | Pre | Post |
| Body weight (kg) | 70.2±3.4 | 70.0±3.2 | 67.2±5.0 | 67.2±5.1 |
| Body fat (%) | 17.0±2.7 | 16.7±2.8 | 16.7±3.2 | 17.5±3.3 |
| Insulin Sensitivty (SI) | 12.6±3.9 | 13.6±5.8 | ||
| HOMA-IR (mU*mmol/L2 | 3.53±0.91 | 2.30±0.72# | 3.93±0.63 | 4.92±1.04 |
| Glucose (mmol/L) | 5.04±0.01 | 4.69±0.26 | 4.68±0.17 | 4.82±0.06 |
All values are expressed as mean±SEM.
SI, insulin sensitivity
HOMA-IR, homeostatic model assessment for insulin resistance
Data missing for one subject
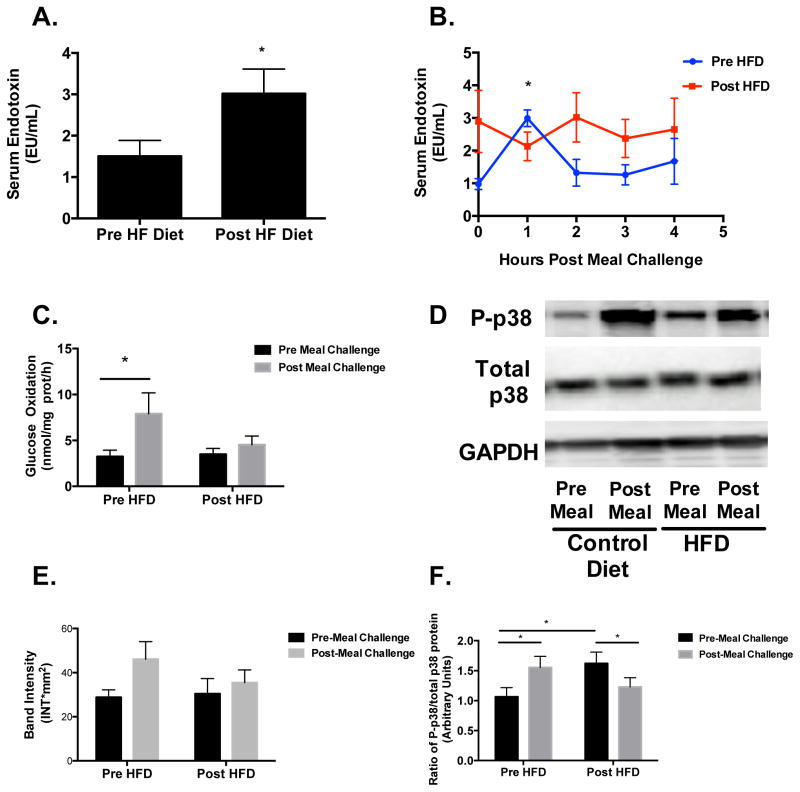
There was a significant increase in fasting endotoxin concentrations following HFD in study 1 and 2 (Figure 1A). In study 2, we observed a significant 2.5-fold increase (P<0.05) in postprandial endotoxin concentrations at 1 hr following the consumption of the high fat meal challenge prior to HFD, which was not evident after the HFD (Figure 1B). In response to the meal challenge after HFD, serum endotoxin did not change from fasting levels (Figure 1B).
Figure 1. Fasting and postprandial endotoxin, skeletal muscle homogenate glucose oxidation, and p38 protein.
(A) Fasting serum endotoxin concentrations were significantly higher (P<0.05) after HFD for studies 1 and 2 combined. (B) Postprandial endotoxin was significantly increased (P<0.05), relative to baseline, 1 hr post meal challenge before HFD, but not after. (C) Glucose oxidation in skeletal muscle homogenates was significantly increased (P<0.05) 4 hr post meal challenge before HFD, but not after. (D&E) p38 MAPK phosphorylation was increased, although non-significant, 4 hr post meal challenge before HFD, but not after. (F) There was a significant HFD x meal challenge interaction (P<0.001) for phospho- to total p38 protein ratio, which was significantly increased (P<0.01) under fasting conditions after the HFD, and in response to meal challenge before HFD, but decreased after. All data are expressed as mean±SEM. *P<0.05.
There were no changes (P>0.05) in glucose oxidation or any enzyme activities in the homogenates in response to the HFD in study 1 (data not shown).
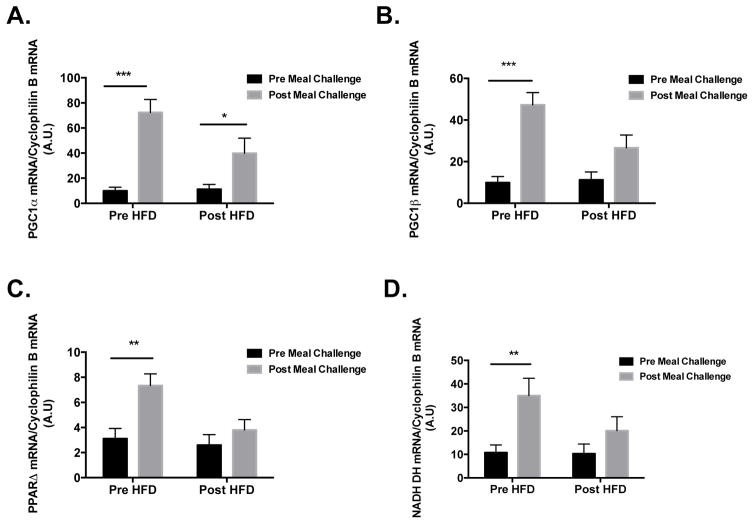
Glucose oxidation was increased (P<0.05) following the meal challenge prior to HFD but not after (Figure 1C). There was a modest, but non-significant, increase in the phosphorylation of p38 in response the meal challenge, which was not evident after the HFD (Figure 1D and E). When comparing the phospho- to total-p38 protein ratio, there was a significant diet x meal challenge interaction (P<0.001) in that the ratio was significantly increased (P<0.05) under fasting conditions after HFD, increased in response to the meal prior to HFD, and then decreased in response to the meal after HFD (Figure 1F). A similar pattern of increases in response to meal challenge prior to HFD was observed with PGC1α, PGC1β, NADH dehydrogenase, and PPARδ mRNA; this response was blunted in all targets after HFD (Figures 2A–D).
Figure 2. Metabolic mRNA targets in skeletal muscle.
mRNA concentrations of PGC1-α (A), PGC-1β (B), NADH dehydrogenase (C), and PPARδ (D) were significantly increased in skeletal muscle 4 h post meal challenge before HFD, all increased significantly less after the HFD. All data are expressed as mean±SEM. *P<0.05, **P<0.01, ***P<0.001.
Discussion
The major finding of the present study is that 5 days of an isocaloric, HFD increases post-absorptive endotoxin concentrations and attenuates the increase in skeletal muscle homogenate glucose oxidation observed during the fasting-to-fed transition. Consistent with the latter observations, the increased phospho- to total-p38 protein ratio, and expression of key metabolic genes during the fasting-to-fed transition were also attenuated following HFD. Importantly, the observed dysregulation of skeletal muscle substrate metabolism occurred in the absence of changes in insulin sensitivity.
The increase in fasting and postprandial endotoxin concentrations is consistent with prior reports in animals (2, 11) and humans (12, 13, 14, 15, 16, 17). However, our study is the first to demonstrate that circulating endotoxin concentrations are increased following 5 days of an isocaloric, HFD in healthy humans. Moreover, this “metabolic endotoxemia” was associated with a blunted postprandial increase in skeletal muscle homogenate glucose oxidation.
There are three isoforms of p38 expressed in skeletal muscle, p38-α, -β, and –γ (18). p38-α is the isoform primarily involved in initiating TLR4 mediated inflammatory signaling (18) and p38-γ has been demonstrated to promote PGC1- α gene transcription (19). Importantly, PGC1-α transcription is an important molecular event regulating mitochondrial oxidative metabolism (20). To our knowledge, we are the first to report in humans these dynamic responses by p38 MAPK in response to acute (a single meal) and chronic (5 days of HFD) feeding. Based on the robust induction of oxidative mRNA targets in response to the meal challenge, we are speculating the increase in the phospho p38 to total p38 ratio is a due to increased phosphorylation of p38-γ. The heightened phospho p38 to total p38 ratio observed in fasting samples after 5 days of HFD is speculated to represent changes in p38-α, which falls in line with the higher serum endotoxin levels. While we acknowledge that the small number of subjects was a limitation to this study, we also believe these data are novel and compelling and warrant future studies to determine which p38 isoforms are responsible for this observation.
In conclusion, we are the first to demonstrate that, in the absence of changes in whole body insulin sensitivity, a short-term HFD increased fasting circulating endotoxin concentrations while resulting in an attenuated increase in circulating endotoxin and skeletal muscle homogenate glucose oxidation during the fasting-to-fed transition. In addition, a short-term HFD alters the dynamics of the phospho-p38 to total p-38 protein ratio under fasting conditions and during the fasting-to-fed transition. Taken together with the smaller increase in mRNA expression, these finding suggest a potential mechanism for the observed dysregulated skeletal muscle substrate metabolism following the fasting-to-fed transition. Importantly, this dysregulated skeletal muscle substrate metabolism was observed prior to measurable changes in whole body insulin sensitivity.
Supplementary Material
What is already known about this subject
Metabolic endotoxemia is associated with obesity and insulin resistance.
High fat feeding in rodents and humans elicits an increase in serum endotoxin.
What does this study add?
Acute (5 days) high fat feeding, under conditions of energy balance, increases fasting serum endotoxin in healthy non-obese males.
Acute (5 days) high fat feeding, under conditions of energy balance, disrupts postprandial metabolic and transcriptional adaptation in skeletal muscle of healthy, non-obese males.
p38 MAPK phosphorylation, relative to total p38 MAPK protein, is increased in skeletal muscle under fasting conditions after 5 days of high fat feeding.
p38 MAPK phosphorylation, relative to total p38 MAPK protein, is increased in skeletal muscle in response to a meal, and this effect is disrupted after 5 days of high fat feeding.
Acknowledgments
AA analyzed data, interpreted data, and wrote the manuscript. KH recruited subjects, conceived study design, and carried out experiments. RM conceived and carried out experiments. KO contributed to study design and planned/provided all meals to the subjects. NB and BD contributed to study design and edited the document. MF, KD, and MH conceived the study design and experiments, and contributed to editing of the document. All authors gave final approval of the submitted and published versions. This research was funded by ADA-07-12 (MWH) and RO1DK078765 (MWH).
Footnotes
Disclosure
The authors declare no conflict of interest.
Competing interests: the authors have no competing interests.
References
- 1.Neves AL, Coelho J, Couto L, Leite-Moreira A, Roncon-Albuquerque R., Jr Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk. Journal of molecular endocrinology. 2013;51:R51–64. doi: 10.1530/JME-13-0079. [DOI] [PubMed] [Google Scholar]
- 2.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 3.Ding S, Lund PK. Role of intestinal inflammation as an early event in obesity and insulin resistance. Current opinion in clinical nutrition and metabolic care. 2011;14:328–333. doi: 10.1097/MCO.0b013e3283478727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisard MI, McMillan RP, Marchand J, Wahlberg KA, Wu Y, Voelker KA, et al. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol Endocrinol Metab. 2010;298:E988–998. doi: 10.1152/ajpendo.00307.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes technology & therapeutics. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 6.Davy BM, Davy KP, Ho RC, Beske SD, Davrath LR, Melby CL. High-fiber oat cereal compared with wheat cereal consumption favorably alters LDL-cholesterol subclass and particle numbers in middle-aged and older men. Am J Clin Nutr. 2002;76:351–358. doi: 10.1093/ajcn/76.2.351. [DOI] [PubMed] [Google Scholar]
- 7.Marinik EL, Frisard MI, Hulver MW, Davy BM, Rivero JM, Savla JS, et al. Angiotensin II receptor blockade and insulin sensitivity in overweight and obese adults with elevated blood pressure. Therapeutic advances in cardiovascular disease. 2013;7:11–20. doi: 10.1177/1753944712471740. [DOI] [PubMed] [Google Scholar]
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 9.Medicine Io. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. 2005. [DOI] [PubMed] [Google Scholar]
- 10.Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obesity research. 2005;13:574–581. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- 11.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PloS one. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laugerette F, Alligier M, Bastard JP, Drai J, Chanseaume E, Lambert-Porcheron S, et al. Overfeeding increases postprandial endotoxemia in men: Inflammatory outcome may depend on LPS transporters LBP and sCD14. Molecular nutrition & food research. 2014 doi: 10.1002/mnfr.201400044. [DOI] [PubMed] [Google Scholar]
- 13.Harte AL, Varma MC, Tripathi G, McGee KC, Al-Daghri NM, Al-Attas OS, et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes care. 2012;35:375–382. doi: 10.2337/dc11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemente-Postigo M, Queipo-Ortuno MI, Murri M, Boto-Ordonez M, Perez-Martinez P, Andres-Lacueva C, et al. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. Journal of lipid research. 2012;53:973–978. doi: 10.1194/jlr.P020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes care. 2011;34:1809–1815. doi: 10.2337/dc10-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 18.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochimica et biophysica acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, et al. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PloS one. 2009;4:e7934. doi: 10.1371/journal.pone.0007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.