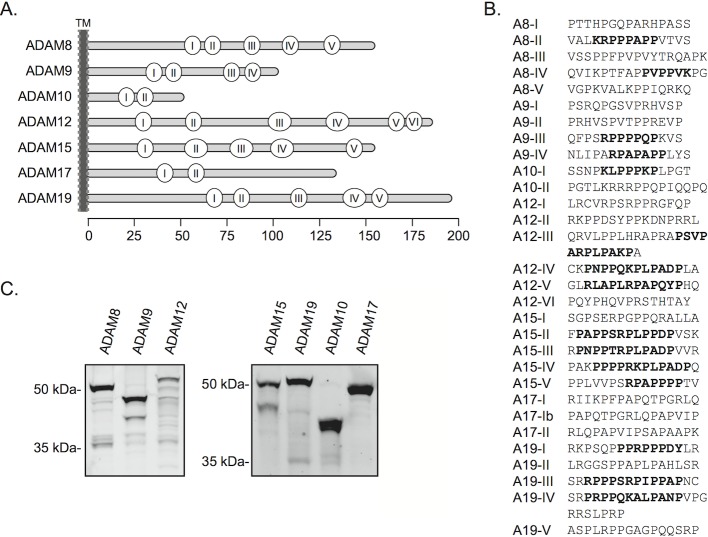
Fig 1. Cytosolic tails of ADAM-metalloproteases and their candidate SH3 binding motifs.
A. Schematic presentation of ADAM cytosolic tails with the location of the candidate SH3-binding proline clusters indicated by circles with roman numerals counting from the transmembrane region to the carboxy terminus. The scale bar indicates distance in amino acid residues. B. Potential SH3 binding sequences within the proline clusters shown in A. Established SH3 target motifs (+ΦPxxP, PxΦPx+, PxxDY, where + is K or R and Φ is a hydrophobic residue) occurring individually or in clusters where they partly overlap each other are indicated in bold. C. Western blotting analysis of the ADAM tails expressed as biotinylated fusion proteins in human 293T cells for use as affinity baits in SH3 domain library screening.