Fig. 4.
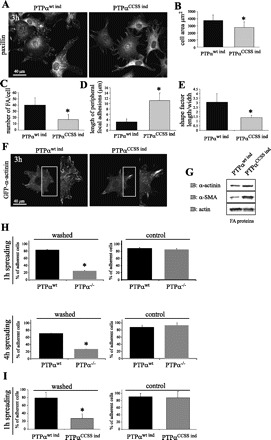
PTPα phosphatase activity is required for FA remodeling. A: genetically modified NIH-3T3 cells lines induced to express wild-type human PTPα (PTPαwt ind) and double-mutant Cys433 → Ser/Cys723 → Ser PTPα (PTPαCCSS ind). Cells were plated on fibronectin for 3 h. FA were observed by staining for paxillin (A) or by transfection with GFP-α-actinin (F). B–E: cell area, number of FA per cell, length of peripheral FA, and shape factor (length vs. width), which indicates degree of polarization, from images in A. Values are means ± SD of 30–40 measurements in 3 independent experiments. *P < 0.01 vs. PTPαwt ind (by unpaired Student's t-test). G: Western blot analysis of FA fraction (FA proteins). Note greater amounts of α-smooth muscle actin (α-SMA) and α-actinin in cells expressing PTPαCCSS ind. Levels of β-actin did not vary considerably and served as control. H and I: PTPα phosphatase activity is required for efficient adhesion to fibronectin. Cell adhesion was measured in primary PTPαwt and PTPα−/− murine fibroblasts (H) and genetically modified NIH-3T3 cells expressing PTPαwt ind and PTPαCCSS ind (I). Cells were stained with calcein-AM and plated on fibronectin for 1 or 4 h and then washed 5 times with PBS. Control cells were not washed. Calcein-AM fluorescence of the remaining cells was measured using a microplate fluorescence reader. Values of intensity were normalized to a percentage of control. Values are means ± SD of 16 tests per cell line in 3 independent experiments. *P < 0.05 vs. PTPαwt ind or PTPαwt.
