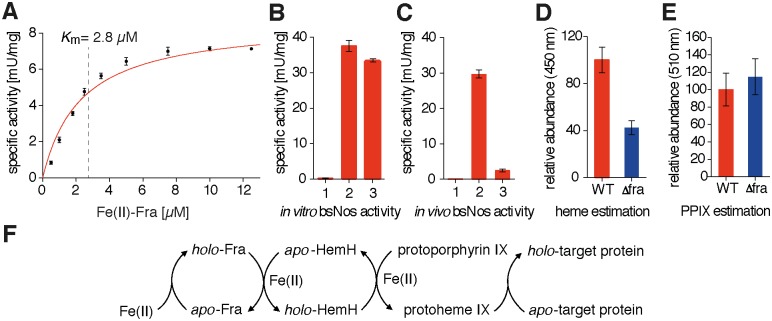
Fig 4. Influence of Fra on the heme maturation pathway.
(A) Kinetics of the conversion of protoporphyrin IX into heme b by HemH. Varying concentrations of Fe2+-charged frataxin as the sole iron source were added in a range of 0.1–10 μM, and conversion of protoporphyrin IX into heme b was followed by fluorescence spectroscopy. Data were fitted according to the Michaelis-Menten model, which resulted in a K m(obs) of about 2.8 ± 0.5 μM and a k cat(obs) of 0.925 ± 0.059 s-1 (error bars represent SEM of three independent experiments). (B) In vitro activities of apo-bsNos (1), holo-bsNos (2) and reconstituted apo-bsNos (3). The reconstitution of apo-bsNos with its heme co-factor was carried out by a coupled transfer assay containing Fe2+-charged Fra, HemH and protoporphyrin IX, and led to a partial restoration of bsNos activity (error bars represent SEM of three independent experiments). (C) In vivo bsNos activities in equalized amounts of total cellular protein. Control of the assay with B. subtilis WT crude protein extract assayed without the addition of N ω-hydroxy-l-arginine (1), with B. subtilis WT crude protein extract (2) and with B. subtilis Δfra crude protein extract (3). The deletion of fra led to a ~12-fold decrease of bsNos activity in the crude mutant cell extract (error bars represent SEM of three independent experiments). (D) Determination of the relative heme contents in B. subtilis WT (red bar) and Δfra (blue bar) cells by acidic acetone extraction and fluorescence analysis of the heme b soret band emission at 450 nm upon excitation at 380 nm. The amount of cellular heme was found to be ~2.5-fold reduced in the Δfra mutant cell (error bars represent SEM of three independent experiments). (E) Determination of the relative protoporphyrin IX concentration in B. subtilis WT (red bar) and Δfra (blue bar) cells by acidic acetone extraction and fluorescence analysis of the protoporphyrin IX soret band emission at 510 nm upon excitation at 410 nm. The amount of cellular protoporphyrin IX was ~1.2-fold elevated in the Δfra mutant cell (error bars represent SEM of two independent experiments). (F) Fra mediated heme b (protoheme IX) maturation and its delivery to heme-dependent target proteins.