Abstract
Objective
The current study marks the first randomized controlled trial to test the benefit of combining Seeking Safety (SS), a present-focused cognitive behavioral therapy for co-occurring posttraumatic stress disorder (PTSD) and alcohol use disorder (AUD), with sertraline, a front-line medication for PTSD shown to also impact drinking outcomes.
Method
Sixty-nine participants (81% female; 59% African American) with primarily childhood sexual (46%) and physical (39%) trauma exposure, and drug dependence in addition to AUD were randomized to receive a partial-dose (12 sessions) of SS with either sertraline (n = 32; M = 7 sessions) or placebo (n = 37; M = 6 sessions). Assessments conducted at baseline, end-of-treatment, 6- and 12-months posttreatment measured PTSD and AUD symptom severity.
Results
Both groups demonstrated significant improvement in PTSD symptoms. The SS plus sertraline group exhibited a significantly greater reduction in PTSD symptoms than the SS plus placebo group at end-of-treatment (M difference = −16.15, p = .04, d = 0.83), which was sustained at 6- and 12-month follow-up (M difference = −13.81, p = .04, d = 0.71, and M difference = −12.72, p = .05, d = 0.65, respectively). Both SS groups improved significantly on AUD severity at all posttreatment time points with no significant differences between SS plus sertraline and SS plus placebo.
Conclusion
Results support the combining of a cognitive behavioral therapy and sertraline for PTSD/AUD. Clinically significant reductions in both PTSD and AUD severity were achieved and sustained through 12-months follow-up, Moreover, greater mean improvement in PTSD symptoms was observed across all follow-up assessments in the SS plus sertraline group.
Keywords: PTSD, alcohol abuse, cognitive-behavioral treatment, sertraline, selective serotonin reuptake inhibitor, randomized clinical trial
Decades of research have documented high rates of trauma exposure among individuals with substance use disorders (SUD; Debell et al., 2014; Torchalla, Nosen, Rostam & Allen, 2012; van Dam, Vedel, Ehring & Emmelkamp, 2012) as well as multiple negative consequences in biological, psychological, and interpersonal domains of functioning (McCauley, Killeen, Gros, Brady & Back, 2012; Norman et al., 2012). Longitudinal studies indicate that for individuals with PTSD, the risk of developing an alcohol use disorder (AUD) or other SUD is approximately six times greater than for those without PTSD (Breslau, Davis, & Schultz, 2003; Creamer, Burgess, & McFarlane, 2001) and among individuals with PTSD, 10 to 61% report alcohol misuse (Debell et al., 2014). This comorbid population seeks treatment more often than alcohol dependent individuals without PTSD, yet the prognosis for treatment is frequently poor (Debell et al., 2014; Ouimette, Ahrens, Moos, & Finney, 1997; Smith & Randall, 2012). Individuals with AUD and comorbid psychopathology are less compliant with treatment, more likely to drop-out, have higher suicide rates, and receive less support for achieving and maintaining sobriety (Smith & Randall, 2012). Additionally, compared to AUD patients without comorbid PTSD, those with comorbid PTSD spend a greater number of hospital overnights for addiction treatment even when there are no differences in substance abuse severity (Brown, Stout, & Mueller, 1999; Sannibale et al., 2013; Smith & Randall, 2012). These findings indicate that the AUD patients with comorbid PTSD have poorer treatment outcomes and may overuse costly inpatient addiction services. PTSD severity is widely reported to worsen early in abstinence (Brady, Killeen, Saladin, Dansky, & Becker, 1994), making treatment of AUD particularly challenging. PTSD symptoms are common triggers of alcohol use relapse that, in turn, can heighten PTSD symptoms (Ralevski, Olivera-Figueroa, & Petrakis, 2014; Coffey et al., 2002; Waldrop, Back, Verduin, & Brady, 2007), creating a maladaptive feedback cycle.
Despite the widespread impression among clinicians and the converging research findings that PTSD comorbidity hampers the treatment responses of individuals in SUD treatments, the two disorders have only more recently been treated simultaneously (Brown, Recupero, & Stout, 1995; Najavits, Weiss, & Leise, 1996; van Dam et al., 2012). Moreover, although cumulative findings suggest the importance of examining treatment-related alcohol outcomes specifically, most clinical trials on PTSD and comorbid SUD have targeted drug-dependent rather than alcohol-dependent individuals. Integrated behavioral interventions for PTSD+SUD are either present-focused or past-focused (Najavits & Hien 2013). Past-focused interventions integrate trauma-focused techniques in tandem with relapse prevention strategies (Mills et al., 2012; Sannibale et al., 2013). Trauma-focused strategies include extensive exploration of trauma memories and in-vivo confrontation of avoided (safe) trauma-reminders (e.g., prolonged exposure). In integrated, present-focused treatment approaches, such as Seeking Safety, there is limited exploration of the trauma memories. Instead, the focus in Seeking Safety is on the impact of traumatic stress on current functioning and its relationship to substance and alcohol use, utilizing psychoeducation and cognitive-behavioral techniques to boost current coping strategies (Najavits & Hien, 2013).
Studies suggest present- and past-focused treatments that address PTSD and SUD simultaneously are more likely to succeed, more cost-effective, and more sensitive to patient needs (Hobbs, Kushner, Lee, Reardon, Maurer, 2011; Mills et al., 2012; Sannibale et al., 2013; Torchalla, Nosen, Rostam & Allen, 2012). Seeking Safety is the most widely tested integrated present-focused treatment to date (i.e., in 20 randomized controlled trials and pilot studies) and has been found to significantly reduce substance use as well as PTSD symptoms across a variety of populations (see Najavits & Hien, 2013). However, more than half of PTSD sufferers continue to have a range of symptoms after receiving treatment, a trend that is consistent with other PTSD treatments (Hien et al., 2009).
Pharmacotherapy plays an increasing role in the treatment of AUD patients specifically. The use of antidepressants in AUD treatment makes sense given the advent of selective serotonin reuptake inhibitors (SSRIs) with excellent safety profiles and the high rates of depression and anxiety disorders that may co-occur with AUDs (e.g., Ralevski, Oliveras-Figueroa, & Petrakis, 2014, Kranzler, Amin, Modesto-Lowe, & Oncken, 1999). In studies of alcohol users without comorbid disorders, findings on the efficacy of SSRIs have been mixed with some studies finding modest but significant reductions (10–26%) in the alcohol consumption of non-clinically depressed heavy drinkers (Naranjo, Kadlec, Sanhueza, Woodley-Remus, & Sellers, 1990; Naranjo et al., 1987; Naranjo et al., 1989) and others finding no effects on alcohol use (Gorelick & Paredes, 1992; Kranzler et al., 1995).
There is compelling evidence supporting the use of SSRIs for PTSD (Friedman, 2013; Ipser & Stein, 2012, Forbes et al., 2010) with findings of significantly greater response to sertraline than placebo leading to FDA approval for both sertraline and paroxetine. However, only one published study has examined the impact of combination SSRI and cognitive behavioral therapy (CBT) among individuals with PTSD and AUD (Brady et al., 2005) with results indicating that pharmacotherapy with CBT was more efficacious than placebo with CBT. In this study, a subgroup of participants with early onset PTSD and less severe AUD who received sertraline demonstrated significantly greater reductions in drinking compared to individuals with early onset and severe AUD. Because the study’s cognitive behavioral component (Project MATCH Research Group, 1997) addressed only AUD symptoms, however, the efficacy of combining sertraline with a CBT that addresses co-occurring PTSD and SUD symptoms remains to be tested.
Research on the classification of AUD into meaningful subtypes has previously identified two clinically distinct groups: a later onset AUD typified by less severity and chronicity of symptoms and an earlier onset presentation with more severe symptomatology (Buydens-Branchey, Branchey, & Noumair, 1989). The claim that SSRI treatment response is moderated by AUD subtypes has been supported by studies reporting significant reduction in alcohol use in later onset AUD treated with sertraline (Pettinati et al., 2000) and poorer drinking outcomes for early onset AUD treated with fluoxetine (Kranzler, Burleson, Brown, & Babor, 1996). However, it remains to be determined whether these subtypes would respond differently to a treatment employing SSRIs and CBT where both AUD and PTSD symptoms are targeted.
Given the existing data suggesting that sertraline 1. decreases alcohol consumption to a modest degree for those with AUD alone; 2. is a first line pharmacotherapy for PTSD; 3. can lead to improvements in drinking and PTSD outcomes among subtypes of individuals with AUD and comorbid depression or PTSD; and 4. has an excellent safety profile, the present study was designed to test the following hypothesis: the combination treatment of Seeking Safety and sertraline would be significantly more efficacious than Seeking Safety and placebo in reducing PTSD and AUD symptoms. An additional exploratory analysis was conducted to examine whether response to treatment was moderated by AUD onset (early vs. late).
Method
All procedures were reviewed and approved by the institutional review boards of The New York State Psychiatric Institute (NYSPI) and The City College of New York. Written informed consent was provided by all participants.
Participants
Inclusion criteria were 1. Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association, 2000) criteria for full PTSD or subthreshold PTSD (Grubaugh et al., 2005) defined as meeting Criteria A (exposure to a traumatic stressor), B (re-experiencing symptoms), E (symptom duration of at least 1 month), and F (significant distress or impairment of functioning), and either C (symptoms of avoidance and/or numbing) or D (increased arousal symptoms) 2. DSM-IV-TR criteria for current alcohol dependence or alcohol abuse with at least 2 heavy drinking days (more than 3 drinks for women and more than 4 drinks for men) in the past 90 days or at least 14 drinks over 30 consecutive days or less than 22 consecutive abstinent days. Individuals who did not meet criteria for alcohol abuse or dependence were eligible if they reported at least one episode of alcohol misuse during the prior 90 days. Alcohol misuse was defined as either hazardous drinking (for women, more than 7 drinks per week; for men, more than 14 drinks per week) or binge drinking (4 or more drinks over a 2 hour time frame for women and 5 or more drinks over a 2 hour time frame for men; NIAAA, 2013).
Exclusion criteria were: 1. advanced stage medical disease as indicated by global physical deterioration and incapacitation, 2. organic mental syndrome 3. diagnosis of bipolar I or psychotic-spectrum disorders 4. any disorder which might have made antidepressant treatment hazardous, 5. current pregnancy or lactation, 6. history of seizures (not related to alcohol withdrawal), 7. current use or prescription of psychotropic medications by another physician, 8. history of allergic reaction to sertraline, 9. current active suicidal or homicidal ideation, intent, or behavior, 10. age over 65 or under 18, and 11. refusal to be audio and videotaped. Individuals with other SUDs or current major depressive disorder were not excluded.
Procedures
Recruitment and pre-randomization assessments
Participants were recruited through newspaper and radio advertisements, flyers, and referrals from outpatient mental health centers between April 2006 and March 2012. Individuals were screened through a brief telephone interview and then completed a baseline interview where alcohol use, PTSD, and demographic data were collected. After baseline assessment and medical clearance, all eligible participants began a one-week, single-blind placebo lead-in phase, during which they met with a trained clinician for a 30–45 minute motivational enhancement session. Those who completed the lead in phase were accepted into the study and randomly assigned to one of the two treatment conditions.
Randomization
Participants, study psychiatrist, therapists, and assessors were blind to treatment condition assignment. The pharmacy created sertraline and matching placebo kits with single-identifier numbers based on a random code that was provided to an unblinded statistician who instructed the psychiatrist how to distribute kits to subjects. An urn randomization procedure was utilized to balance treatment conditions on baseline severity of alcohol and other substance use and baseline severity of depression. Allocation was concealed from study staff for the length of patient’s study participation.
Treatments
1. Integrated, Present-Focused Cognitive Behavioral Therapy
Seeking Safety (Najavits, 2002) is a manualized intervention, based on five central ideas: 1.) safety as the priority; 2.) integrated treatment of PTSD and SUD; 3.) a focus on ideals; 4.) four content areas: cognitive, behavioral, interpersonal, and case management; and 5.) attention to therapist processes. Session content was structured to engage in themes relevant to both PTSD and SUD, and to learning a specific CBT skill. In consultation with its developer, Lisa Najavits, Ph.D., the Seeking Safety treatment was abbreviated from 25 to 12 core sessions to better fit within a feasible time-frame for community-based outpatient treatment programs. Treatment sessions were delivered in a 60-minute weekly individual format by eight experienced (PhD or LCSW level) research therapists who underwent rigorous training in the Seeking Safety protocol which included a 3-day introduction to the treatment manual followed by delivery of a test case in which all sessions were reviewed by the expert supervisor for adherence and competence, and ongoing weekly supervision and case review during the study period.
2. Medication
Matching capsules contained sertraline or placebo as well as riboflavin to assess medication adherence. Compliance was also monitored by pill count. Participants receiving sertraline started on 50 mg daily and titrated up to 200 mg daily over a 2-week period. Participants continued on their full sertraline dose until the end of the trial and were tapered after unblinding. Responders were offered the option to remain on medication.
Supervision and fidelity
All Seeking Safety sessions were audiotaped and a proportion (>50%) of session recordings reviewed and rated using the Seeking Safety Session Format Checklist (Najavits, 2003) by an expert supervisor (Lisa Litt, Ph.D.) for curriculum adherence. Throughout the course of the study research therapists met weekly for supervision. If curriculum adherence fell below the competency criterion, more supervision was provided. A randomly selected 25% of sessions reviewed by the supervisor were also rated by one of the lead investigators as a means of confirming supervisor fidelity and interrater reliability. Supervisor fidelity was defined as agreement at a 70% level with specific adherence measures for the Seeking Safety curriculum.
Measures
During the intervention phase of the study, participants met weekly with a research assistant for the collection of a urine sample, alcohol breathalyzer test, and self-report assessments of PTSD symptoms, alcohol and drug use, and any adverse events. After the study treatment phase, assessment interviews were conducted by blind independent assessors at end-of treatment, 6- and 12-months posttreatment.
Sociodemographics
Age, race/ethnicity, education, marital status, employment pattern, and prior AUD treatment episodes were collected at the baseline assessment. See Table 1.
Table 1.
Baseline Demographic and Diagnostic Characteristics by Treatment Group (N = 69).
| Characteristic | Seeking Safety + Sertraline (n = 32) |
Seeking Safety + Placebo (n = 37) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Women | 26 | 81.3 | 30 | 81.1 |
| Race/ethnicity | ||||
| African American | 16 | 50.0 | 25 | 67.6 |
| Caucasian | 10 | 31.3 | 6 | 16.2 |
| Latina/o | 3 | 9.4 | 4 | 10.8 |
| Other | 3 | 9.4 | 2 | 5.4 |
| Marital Status | ||||
| Married | 9 | 28.1 | 5 | 13.5 |
| Single | 17 | 53.1 | 25 | 67.6 |
| Divorced/separated/widowed | 6 | 18.8 | 7 | 18.9 |
| Employment | ||||
| Employed | 23 | 71.9 | 30 | 81.1 |
| Unemployed | 8 | 25.0 | 4 | 10.8 |
| Student/retired/disabled | 1 | 3.1 | 3 | 8.1 |
| Past 7-day abstinence rate | 3 | 9.7 | 4 | 10.8 |
| Alcohol Dependence | 28 | 87.5 | 33 | 89.2 |
| Alcohol Abuse | 3 | 9.4 | 0 | 0 |
| Early onset AUD | 13 | 40.6 | 16 | 48.5 |
| Drug Dependence | ||||
| Cannabis | 5 | 15.6 | 3 | 8.1 |
| Cocaine | 8 | 25.0 | 13 | 35.1 |
| Comorbid AUD and SUD | 16 | 50.0 | 22 | 59.5 |
| Lifetime traumatic experiences* | ||||
| Child physical | 14 | 43.3 | 18 | 48.5 |
| Adult physical | 16 | 50.0 | 16 | 42.4 |
| Child sexual | 12 | 36.7 | 15 | 41.2 |
| Adult sexual | 12 | 36.7 | 13 | 35.3 |
| Transportation accident | 19 | 60.0 | 27 | 73.5 |
| Life-threatening illness | 7 | 23.3 | 8 | 20.6 |
| Exposed to violent death | 14 | 43.3 | 10 | 26.5 |
| Current Major Depression | 20 | 62.5 | 22 | 59.5 |
| M | SD | M | SD | |
| Age (years) | 42.2 | 9.8 | 42.5 | 8.5 |
| Education (years) | 13.7 | 3.1 | 13.0 | 2.0 |
| Age at PTSD onset | 28.1 | 14.4 | 22.8 | 13.5 |
| CAPS severity, total | 65.8 | 19.4 | 59.0 | 19.2 |
| DDD** | 6.8 | 5.1 | 6.9 | 4.7 |
| HDD** | 3.3 | 2.2 | 2.9 | 2.4 |
| Prior alcohol treatment episodes | 1.1 | 1.9 | 1.6 | 4.3 |
Note. PTSD = posttraumatic stress disorder; DDD = drinks per drinking day; HDD = heavy drinking day (5+ drinks for men, 4+ for women); CAPS = Clinician Administered PTSD Scale; AUD = alcohol use disorder (abuse or dependence); SUD = substance use disorder (abuse or dependence).
No participants endorsed natural disaster event.
in past 7 days.
Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995)
The CAPS is a structured, clinical interview for assessing the frequency and intensity of DSM-IV-TR PTSD symptoms, impairments in social and occupational functioning, diagnosis, and overall symptom severity. The scale consists of the Re-Experiencing, Avoidance/Numbing, and Hyperarousal symptom cluster subscales. The frequency and intensity scores for each symptom cluster subscale are summed to obtain an overall total scale score. CAPS were administered by trained Master’s or PhD level clinicians, and scoring data demonstrated good to excellent internal consistency reliability across baseline and all follow-up assessments (α = .86 – .92).
Structured Clinical Interview for DSM-IV for Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 2002) modules for Mood Disorders, Alcohol and Psychoactive Substance Use Disorders, and Psychotic Disorder screen. The SCID-I, a semi-structured interview, was administered at baseline and follow-up points to assess current AUD/SUD diagnoses, age of AUD/SUD onset, and the presence of any other current or past mood disorder [e.g., major depressive disorder or dysthymic disorder). Axis II (personality) disorders were not assessed. AUD/SUD diagnoses were considered current if diagnostic criteria were met in the prior 6 months. High inter-rater reliability has been demonstrated for the SCID-I (First et al., 2002).
Timeline Follow-Back (TLFB; Sobell & Sobell, 1992)
TLFB was used to assess alcohol use patterns before the start of treatment, weekly during the trial, and at each follow-up timepoint. Participants retrospectively estimated their daily alcohol consumption in the previous 90 days with a detailed calendar to help orient them to patterns in their drinking and specific episodes of erratic or binge drinking. TLFB has demonstrated good reliability as an instrument for the estimation of daily alcohol consumption (Sobell, Sobell, Leo, & Cancilla, 1988).
An alcohol breathalyzer test was administered at all study visits in order to measure participants' blood alcohol concentration. Urine toxicology tested for the presence of cocaine, opiates, methadone, cannabis, phencyclidine and amphetamines at all assessment timepoints. In addition, two pregnancy tests were conducted at baseline and week six of treatment. Urine samples were also tested for riboflavin to assess medication compliance.
Participants were compensated $30 for the completion of baseline, end-of-treatment, and follow-up assessments. They received $15 at each treatment session with the return of their pill-bottles and completion of weekly assessments.
Statistical Methods
Bivariate analyses were utilized to compare demographics and baseline symptom severity between the combined Seeking Safety with sertraline group (SS+Sertraline) and the Seeking Safety with placebo group (SS+Placebo), and to explore the data for potential covariates for the main omnibus analyses. The main outcome variable for PTSD was CAPS total score, which is comprised of the sum of frequency and intensity ratings across 17 symptoms, and was administered at baseline and all follow-up assessments. The main outcome variables for AUD were average number of drinks per drinking day in the past 7 days (DDD), number of heavy drinking days in the past 7 days (HDD; five or more drinks per day for men and four or more drinks per day for women are considered heavy drinking days), and self-reported abstinence from alcohol in the prior 7 days and negative breathalyzer tests at follow-up assessments.
All analyses were conducted on the intent-to-treat sample. Generalized estimating equations (GEE) were utilized to model PTSD and drinking outcomes (Ballinger, 2004). This method is an extension of the generalized linear model that handles correlated data arising from repeated measurements, requires no parametric distribution assumption, and provides robust inference with respect to misspecification of the within-subject correlation (Zeger & Liang, 1986; Zeger, Liang, & Albert, 1988). A temporal within-subjects autoregressive [AR(1)] correlation matrix was used to model participants across timepoints. Models were specified according to the distributions of the outcome measures. For example, identity link functions for normal distributions were used to model CAPS severity scores, negative binomial models with log link were applied to the alcohol consumption measures of HDD and DDD, and past 7 days abstinence rate was modeled using logit link for binary distribution. Accordingly, results are reported using parameter estimates for CAPS, incidence rate ratios for HDD and DDD, and odds ratios for abstinence rate.
All models included variables of time, treatment, time-by-treatment interaction, and any demographic or baseline diagnostic covariates for which there was a significant difference between groups. Consistent with prior studies applying similar analytic methods to comparable sample sizes (Schneier et al., 2012), and to reduce the probability of Type-II errors (Selvin, 1996), interactions that were at least trend-level (i.e., α < .10) were probed for simple effects at end-of-treatment and follow-up timepoints. When an interaction did not meet this criterion, outcomes were modeled as main effects with covariates of time and baseline values of the outcome measures included in the model. All simple and main effects were considered significant at the α = .05 level (two-tailed). Since three drinking variables were analyzed, Bonferroni corrections were applied to all models of AUD outcomes in order to control for Type I error. Sensitivity analyses with multiple imputation were conducted to further assess the influence of missing data in models that were significant.
Additional exploratory analyses were conducted to examine differences in treatment effects associated with age of AUD onset. Based on evidence that subtyping of AUD by age of onset may moderate medication effects, 25 was utilized as the study’s cut-off for early vs. late onset AUD classification (Roache, Wang, Ait-Daoud, & Johnson, 2008; Kranzler, Feinn, Armeli, & Tennen, 2012). AUD age of onset was ascertained from the SCID. Three way interactions of time-by-treatment-by-AUD subtype were probed for simple effects if they approached significance at the α = .10 level, and simple effects were considered significant at the α = .05 level.
Results
Demographic and Baseline Characteristics
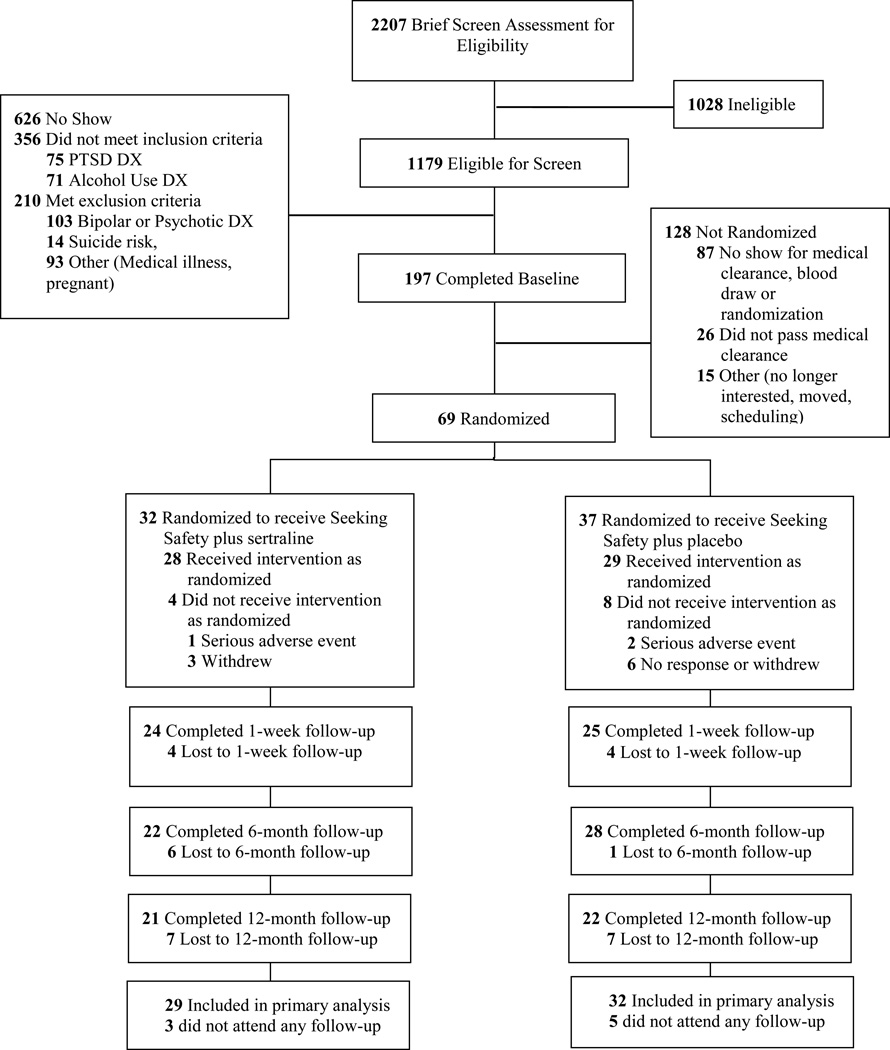
Figure 1 illustrates the participant flow from initial eligibility screening through 6-month follow-up. A total of 69 individuals were randomized into the study. Table 1 presents baseline demographic and descriptive characteristics. No differences were found between treatment conditions with regard to alcohol use frequency/severity, PTSD severity, other SUD comorbidities, or demographic characteristics. Three participants were removed due to serious medical illness. These incidents were reported to the study’s institutional review boards and none were determined to be study-related.
Figure 1.
CONSORT Diagram of participant flow through the protocol. PTSD = posttraumatic stress disorder; DX = diagnosis.
Treatment Adherence
Adherence to medication as measured by riboflavin levels in weekly urine collection did not differ between treatment conditions, χ2(1) = 0.77, p = .44. Rates of riboflavin detection during treatment period for SS+Sertraline and SS-Placebo were 46% and 40% respectively. Overall, 91% attended at least one medication visit and 90% attended at least one Seeking Safety session. Sixty-one participants (88%) attended at least one follow-up assessment. The eight participants who missed all three follow-up assessments could not be included in the outcome models. Attendance rates of Seeking Safety sessions were equivalent across treatment groups [SS+Sertraline: M = 6.7, SD = 4.0; SS+Placebo: M = 6.0, SD = 4.3; t(67) = 0.71; p = .48]. Study retention rates did not differ across treatment groups [χ2(1) = 0.05; p = .83]; 59.4% of SS+Sertraline and 56.8% of SS+Placebo attended at least half of treatment (six or more therapy sessions and six or more medication visits).
PTSD Outcome
Table 2 depicts PTSD severity scores at baseline, end-of-treatment, 6- and 12-month follow-up. The final model for PTSD outcome included time, treatment type, and a time-by-treatment interaction term. Not all participants attended all three follow-up assessments (end-of-treatment, 6- and 12-month follow-ups), and preliminary data analyses indicated that across each of the dependent variables there was not sufficient evidence to conclude that data was not missing completely at random (all ps > .20; Little’s MCAR test; Little, 1988). Under this assumption, GEEs are equipped to handle missing data without compromising parameter estimates (Ballinger, 2004). Significant decreases in CAPS scores were observed in both groups from baseline to end-of-treatment (SS+Sertraline: M difference = −32.79, CI95: −43.38 to −22.20, p < .001; SS+Placebo: M difference = −16.70, CI95: −27.38 to −6.02, p = .002) which were sustained at 6-month follow-up (SS+Sertraline: M difference = −35.20, CI95: −44.80 to −25.60, p < .001; SS+Placebo: M difference = −20.92, CI95: −29.71 to −12.12, p < .001) and 12-month follow-up (SS+Sertraline: M difference = −42.22, CI95: −50.48 to −33.96, p < .001; SS+Placebo: M difference = −29.30, −38.97 to −19.63, p < .001).
Table 2.
Observed means of PTSD and alcohol use outcomes at baseline, end-of-treatment and 6 and 12 month follow-up with model-based treatment effects.
| Outcome | Seeking Safety + Sertraline (n = 32) |
Seeking Safety + Placebo (n = 37) |
Treatment Group Effect (SS+Sertraline vs. SS+Placebo) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CAPS total | n | M | SD | n | M | SD | Estimate | 95% CI | p |
| Baseline | 32 | 65.50 | 20.03 | 37 | 59.50 | 18.97 | - | - | - |
| End-of-treatment | 24 | 36.25 | 28.23 | 25 | 41.88 | 29.30 | −16.15 | −31.18, −1.13 | .04 |
| 6-month | 21 | 30.09 | 20.70 | 28 | 37.46 | 25.88 | −13.81 | −26.88, −0.74 | .04 |
| 12-month | 21 | 24.90 | 19.95 | 22 | 31.82 | 24.44 | −12.72 | −25.40, −0.03 | .05 |
| Drinking | n | M | SD | n | M | SD | IRR | 95% CI | p |
| HDD | 1.60 | 0.61, 4.23 | .34 | ||||||
| Baseline | 32 | 3.13 | 2.17 | 37 | 2.89 | 2.35 | |||
| End-of-treatment | 22 | 1.05 | 1.79 | 25 | 0.48 | 1.69 | |||
| 6-month | 22 | 0.86 | 1.46 | 28 | 0.75 | 1.53 | |||
| 12-month | 20 | 0.30 | 0.47 | 21 | 0.24 | 0.44 | |||
| DDD | 1.38 | 0.63, 3.04 | .42 | ||||||
| Baseline | 32 | 7.03 | 5.00 | 37 | 6.89 | 4.69 | |||
| End-of-treatment | 22 | 2.45 | 3.00 | 25 | 1.40 | 2.52 | |||
| 6-month | 22 | 2.41 | 3.06 | 28 | 3.14 | 4.84 | |||
| 12-month | 20 | 2.55 | 3.01 | 21 | 2.62 | 4.63 | |||
| n | % | n | % | OR | 95% CI | p | |||
| Abstinence | 1.54 | 0.62, 3.83 | .35 | ||||||
| Baseline | 32 | 9.40 | 37 | 10.80 | |||||
| End-of-treatment | 22 | 45.50 | 25 | 60.00 | |||||
| 6-month | 22 | 54.50 | 28 | 46.40 | |||||
| 12-month | 20 | 40.00 | 21 | 57.10 | |||||
Note. PTSD = Post-traumatic stress disorder; CAPS = Clinician Administered PTSD Scale; DDD = drinks drinking days; HDD = heavy drinking days; IRR = incidence rate ratio; OR = odds ratio; CI = confidence interval. Drinking outcomes are for previous 7 days. PTSD outcomes were probed at each timepoint after trend-level time-by-treatment interaction. Drinking outcomes were modeled for main treatment effects after no interactions were observed.
To examine differences between groups, a trend-level time-by-treatment interaction (p = .096) was probed for simple effects, which revealed a significantly greater reduction in CAPS scores at end-of-treatment in the SS+Sertraline group relative to the SS+Placebo group (M difference = −16.15, CI95: −31.18 to −1.13, p = .035, d = 0.83), as well as 6-month follow-up (M difference = −13.81, CI95: −26.88 to −0.74, p = .038, d = 0.71) and 12-month follow-up (M difference = −12.72, CI95: −25.40 to −0.03, p = .049, d = 0.65). Effect sizes were calculated following guidelines for models employing GEEs (Feingold, 2009), and can be characterized as large for end-of-treatment, and medium for 6- and 12-month follow-ups (Cohen, 1977). Although the impact of missing data cannot be fully ascertained, a comparison of results from the GEE model (which uses maximum likelihood estimation) with another recommended approach (Hallgren & Witkiewitz, 2013) utilizing multiple imputation demonstrated comparable parameter estimates in the pooled results of five imputed datasets (end-of-treatment: M difference = −16.86, CI95: −30.21 to −3.52, p = .013 ; 6-month follow-up: M difference = −14.61, CI95: −27.83 to −1.38, p = .031; 12-month follow-up: M difference = −13.15, CI95: −24.27 to −2.04, p = .020).
These results were further underscored by differences between the groups in the amount of participants who achieved clinically significant change, as defined by a 15-point drop on CAPS total score (Weathers, Keane & Davidson, 2001). At end-of-treatment, 79% of SS+Sertraline patients met this criterion, compared to 48% in the SS+Placebo group, highlighting the significant advantage of combined treatment, χ2(1) = 5.12, p = .02. At 6-month follow the SS+Sertraline group maintained a higher rate of improvement relative to the SS+Placebo group (82% vs. 64%) but this difference was no longer significant, χ2(1) = 1.89, p = .17. Seeking Safety + Sertraline’s relative advantage over SS+Placebo was most pronounced at 12-month follow-up where the SS+Sertraline group exhibited a significantly higher rate of clinically significant change than the SS+Placebo group, 95% vs. 64%, χ2(1) = 6.48, p = .01.
Alcohol Use Outcomes
Table 2 displays alcohol use outcomes (based on weekly assessment measures) at baseline, end-of-treatment, 6- and 12- month follow-ups. At end-of-treatment and both follow-up periods, both treatment groups exhibited significant reductions in all drinking outcomes (adjusted for multiple comparisons). Decreases were observed in DDD at end-of-treatment (SS+Sertraline: IRR = 0.32, CI95: 0.18 to 0.57, p < .001; SS+Placebo: IRR = 0.22, CI95: 0.11 to 0.46, p < .001) at 6-month follow-up (SS+Sertraline: IRR = 0.35, CI95: 0.19 to 0.64, p = .003; SS+Placebo: IRR = 0.48, CI95: 0.27 to 0.84, p = .03), and 12-month follow-up (SS+Sertraline: IRR = 0.34, CI95: 0.20 to 0.59, p < .001; SS+Placebo: IRR = 0.38, CI95: 0.19 to 0.76, p = .02), number of HDD at end-of-treatment (SS+Sertraline: IRR = 0.33, CI95: 0.16 to 0.67, p = .006; SS+Placebo: IRR = 0.19, CI95: 0.05 to 0.68, p = .03), at 6-month follow-up (SS+Sertraline: IRR = 0.27, CI95: 0.13 to 0.54, p < .001; SS+Placebo: IRR = 0.27, CI95: 0.12 to 0.60, p = .003), and 12-month follow-up (SS+Sertraline: IRR = 0.09, CI95: 0.04 to 0.20, p < .001; SS+Placebo: IRR = 0.09, CI95: 0.04 to 0.21, p < .001), and seven day abstinence rate at end-of-treatment (SS+Sertraline: OR = 0.09, CI95: 0.02 to 0.49, p = .02; SS+Placebo: OR = 0.11, CI95: 0.04 to 0.33, p < .001), at 6-month follow-up (SS+Sertraline: OR = 0.06, CI95: 0.01 to 0.34, p = .003; SS+Placebo: OR = 0.16, CI95: 0.05 to 0.50, p = .006), and at 12-month follow-up (SS+Sertraline: OR = 0.12, CI95: 0.02 to 0.60, p = .03; SS+Placebo: OR = 0.09, CI95: 0.03 to 0.29, p < .001). However, between-groups analyses revealed no significant time-by-treatment interaction or overall main effect of the addition of sertraline to SS treatment on any of the alcohol outcome variables.
Exploratory AUD subtype analysis
Participants were categorized as early (n = 29) or late (n = 36) onset AUD based on their age of onset of alcohol abuse or dependence (25 or less for early, over 25 for late). Four participants could not be categorized due to missing age data. Mean age of AUD onset was 19.1 (SD = 3.0) in the early subgroup and 37.0 (SD = 8.6) in the late subgroup. Mean age of PTSD onset was 20.6 (SD = 13.0) in the early onset AUD subgroup and 29.3 (SD = 14.2) in the late onset AUD subgroup. Both of these age means were significantly different between groups [AUD onset: t(63) = −10.6, p < .001; PTSD onset: t(63) = −2.6, p = .01]. However, none of the baseline symptom characteristics of either disorder were significantly different between groups [CAPS: early onset AUD M = 62.7, SD = 19.6 vs. late onset AUD M = 62.9, SD = 20.4, t(63) = −0.03, p = .98; DDD: early onset AUD M = 6.6, SD 4.5 vs. late onset AUD M = 7.5, SD = 5.2, Kolmogorov-Smirnov (KS; nonparametric comparison) z = 0.73, p = .66; HDD: early onset AUD M = 3.0, SD = 2.1 vs. late onset AUD M = 2.9, SD = 2.2, KS z = .28, p = .99; abstinence: early onset AUD 11.1% abstinence rate vs. late onset AUD 10.3% abstinence rate, χ2(1) = 0.01, p = .92].
In the SS+Sertraline group, 41% of participants were categorized as early onset AUD, and in the SS+Placebo group, 48% of participants were categorized as early onset AUD. Preliminary analyses indicated that there were no significant differences in the proportion of AUD subtypes in each treatment condition, χ2(1) = 0.41, p = .52. To explore potential differences between early and late AUD onset to sertraline treatment, the main outcome models were rerun with three-way time-by-treatment-by-subgroup interaction terms. However, this interaction was not significant in any of the models (all ps > .10) suggesting that there were no differences between the treatments in how individuals with early vs. late AUD onset changed over time.
Discussion
The current study marks the first randomized controlled trial to test the benefit of combining Seeking Safety, an integrated, present-focused CBT for the treatment of co-occurring PTSD and AUD and sertraline, a front-line medication for PTSD which has been shown to also impact drinking outcomes. Overall, clinically significant pre- to post- mean improvements in PTSD symptoms and on all drinking outcomes were observed in both treatment arms that received Seeking Safety. Further, results demonstrated that adding sertraline to Seeking Safety significantly improved PTSD symptom reductions among individuals with co-occurring AUD or alcohol misuse in comparison to Seeking Safety with placebo. The addition of sertraline did not confer any further benefits to the psychotherapy for any of the alcohol use outcomes. Study strengths included: the overall advantages of a rigorous randomized controlled trial with high internal and external validity; inclusion of a wide range of AUD and PTSD symptom severity including subthreshold PTSD that suggests treatment gains were not simply a regression to the mean; no requirement for abstinence during the lead-in phase; excellent treatment (i.e., Seeking Safety) fidelity; and a heterogeneous sample of racial/ethnic minorities who are typically underrepresented in alcohol clinical trials.
The impact of co-occurring PTSD and AUD on treatment entry, retention, and outcome has been broadly recognized and represent significant challenges to the effective recovery of both disorders. Research from the past decade has underscored the utility of addressing these disorders concurrently and from an integrative approach (Hien et al., 2009; Mills et al., 2012; Smith & Randall, 2012; van Dam et al., 2012). Nevertheless, investigations that combine effective pharmacological and cognitive behavioral therapies for comorbid PTSD and AUD, in contrast to those which have examined PTSD and SUD, have been sparse (Foa et al., 2013; Ralevski, Olivera-Figueroa & Petrakis, 2014; Sannibale et al., 2013), with few published trials and only one (Sannibale et al., 2013) to date that employs an integrative cognitive behavioral approach to traumatic stress and alcohol misuse.
Although on average, study participants in both conditions improved in their PTSD outcomes, those who received sertraline in combination with Seeking Safety demonstrated a 16 point advantage in their CAPS score reduction at the end-of-treatment and achieved clinically significant improvements in PTSD symptoms beyond those achieved by those receiving Seeking Safety and placebo, which were sustained through the 12-month follow-up (95% versus 64%). Notably, more than half of those who received Seeking Safety and placebo also achieved high levels of symptom reduction in both the PTSD and AUD domains. Given that these kinds of symptoms can be so intractable (e.g., people with PTSD describe living with their unrelenting, haunting memories and other symptoms for years past the original trauma), chronically interfering with daily functioning, the importance of reductions at this level over the course of time should be emphasized. Indeed, Foa and colleagues’ (2013) recent study of naltrexone in combination with prolonged exposure did not demonstrate a significant impact of exposure therapy (another type of CBT that focuses on processing past traumatic experiences) on PTSD among those with PTSD/AUD. We speculate that the addition of sertraline may have facilitated patients’ ability to derive greater benefits from their psychotherapy, corroborating commonly held clinical wisdom suggesting that psychotropic medications promote a patient’s ability to use psychotherapy more effectively. This is noteworthy, given that sertraline has only been tested for comorbid PTSD and AUD in one other study (Brady et al., 2005), where only a trend-level effect on PTSD symptoms was observed.
Seeking Safety, with and without sertraline, was associated with reductions in alcohol use frequency and severity at end of treatment, which was sustained at 6- and 12- month follow-ups, underscoring the benefits of cognitive behavioral strategies for addressing safe coping in this comorbid population. This is particularly striking given that there was no requirement for abstinence during the lead in phase. Moreover, there was no additional effect of sertraline on drinking outcomes. The literature has been mixed regarding the use of SSRIs in the treatment of heavy or regular drinking, particularly among those with comorbid conditions such as PTSD or depression (e.g., Ralevski, Olivera-Figueroa & Petrakis, 2014; Ipser & Stein, 2012). Reviews of controlled medication trials for comorbid AUD and depression revealed that antidepressants decreased drinking in only 38% of studies compared to the reduction of depressive symptoms in 75% of studies (Pettinati, 2004). Among the few studies treating comorbid PTSD and AUD with antidepressants, sertraline improved drinking outcomes in the less severe, late AUD onset subtype (Brady et al., 2005). In a more recent study, Petrakis and colleagues (2012) reported no effects of the SSRI paroxetine on alcohol outcomes.
Notably, the present findings showed no evidence of any negative alcohol response from combining sertraline with Seeking Safety. Indeed, the main effect analyses revealed that both treatment conditions were associated with significant reductions in PTSD symptoms and alcohol consumption rates from baseline to end-of-treatment; these were sustained over the follow-up periods. These findings are in line with a growing body of psychotherapy research in the area of trauma and addiction comorbidity conducted over the past twenty years, which clearly demonstrates that integrative cognitive behavioral interventions for PTSD and substance use–like Seeking Safety—can have an impact on both disorders (Najavits & Hien, 2013, Dass-Brailsford & Myrick, 2010; Torchalla, Nosen, Rostam, & Allen, 2012; van Dam et al., 2012). In the present study, clinically significant reductions in both alcohol use and PTSD symptom frequency and severity at the end of twelve weeks of treatment, sustained through the 12-month follow-ups, highlight the benefit of the Seeking Safety intervention.
Seeking Safety with placebo or sertraline also worked equally well with both early and late onset AUD. Although there is literature suggesting differential responses to SSRIs between early and late onset AUD (e.g., Brady et al., 2005; Jonas et al., 2014), our exploratory analysis found no evidence of this in the sample’s PTSD or AUD outcomes. Nevertheless, the role of AUD typologies in SSRI treatment response requires further examination. Kranzler et al. (2011) recently investigated the contribution of genotypic polymorphism at a prime serotonin transporter site and found that the moderating effect of AUD age of onset on SSRI treatment outcome was only present in late onset AUD possessing a specific allele variation (L’ homozygotes at 5-HTTLPR). If sertraline response is predicated on a subgroup within those with late onset AUD, it is possible that the study’s sample size may have limited the ability to detect differential sertraline effects on drinking outcomes among its identified AUD subtypes.
That placebo did not outperform sertraline on alcohol outcomes must be interpreted in the context of several limitations including the single-site context and modest sample size, particularly with regards to men, who represented less than 20% of the sample. Indeed, with regards to comorbid PTSD and AUD in men, this study should be considered hypothesis-generating rather than hypotheses-confirming. A ceiling effect on alcohol treatment gains, particularly with respect to heavy drinking days, may have obscured any additional advantage sertraline conferred over and above Seeking Safety. Moreover, since all participants also received one session of motivational enhancement therapy (MET), disentangling the benefits of Seeking Safety from MET without a no-treatment control group is not possible. The interpretability of these findings would have been enhanced by a medication-only and/or comparative behavioral treatment arm, but the added benefits of such a design must be weighed against its considerable financial and recruitment burden. Findings must also take participant attrition into account, as well as modest medication adherence, although analyses did not reveal differential attrition or adherence by treatment condition.
In sum, results suggest that adding sertraline to an integrated, present-focused cognitive behavioral treatment of co-occurring PTSD and AUD can lead to synergistic benefits for PTSD. The significant reductions of PTSD symptom severity and drinking experienced across both treatment conditions at end-of-treatment and sustained over 6- and 12-month follow-up provide further support to extant research on the benefit of Seeking Safety (e.g., see for example van Dam et al. for a review of this literature) in amelioration of SUD outcomes. Since PTSD improvements have been shown to lead to later substance use improvements (Hien et al., 2010; Mills et al., 2012; Morgan-Lopez et. al, 2014), these findings provide a positive indication for the use of sertraline in the treatment of PTSD among those with AUD.
Public health significance.
For individuals with PTSD and AUD, this study demonstrated that the combination of Seeking Safety, a present-focused trauma therapy, and sertraline, enhanced PTSD symptom reduction when compared to Seeking Safety and placebo. Drinking outcomes were significantly improved with and without sertraline. These findings suggest the benefit of an integrated cognitive behavioral treatment and SSRI approach to co-occurring PTSD and AUD.
Acknowledgments
This study was supported by grant R01AA014341 from the National Institute on Alcohol Abuse and Alcoholism (primary investigator: Denise A. Hien).
Dr. Levin currently receives medication from US WorldMed for an ongoing study that is sponsored by the National Institute on Drug Abuse and served as a consultant to GW Pharmaceuticals, Eli Lily, and served on an advisory board to Shire in 2006–2007.
Contributor Information
Denise Hien, Department of Psychology, The City College of New York, The City University of New York; Department of Psychiatry, Columbia University College of Physicians and Surgeons, and New York State Psychiatric Institute.
Frances Rudnick Levin, Columbia University College of Physicians and Surgeons and New York State Psychiatric Institute.
Lesia Ruglass, Department of Psychology, The City College of New York, The City University of New York.
Teresa López-Castro, Department of Psychology, The City College of New York, The City University of New York.
Santiago Papini, The City College of New York, The City University of New York & New York State Psychiatric Institute.
Mei Chen Hu, Columbia University College of Physicians and Surgeons and New York State Psychiatric Institute.
Lisa Cohen, Columbia University College of Physicians and Surgeons and New York State Psychiatric Institute.
Abigail Herron, Columbia University College of Physicians and Surgeons and New York State Psychiatric Institute.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organizational Research Methods. 2004;7(2):127–150. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brady KT, Killeen T, Saladin ME, Dansky B, Becker S. Comorbid substance abuse and posttraumatic stress disorder: Characteristics of women in treatment. The American Journal on Addictions. 1994;3(2):160–164. [Google Scholar]
- Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research. 2005;29(3):395–401. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry. 2003;60(3):289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Recupero PR, Stout R. PTSD substance abuse comorbidity and treatment utilization. Addictive Behaviors. 1995;20(2):251–254. doi: 10.1016/0306-4603(94)00060-3. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Stout RL, Mueller T. Substance use disorder and posttraumatic stress disorder comorbidity: Addiction and psychiatric treatment rates. Psychology of Addictive Behaviors. 1999;13(2):115–122. [Google Scholar]
- Buydens-Branchey L, Branchey MH, Noumair D. Age of alcoholism onset. I. Relationship to psychopathology. Archives of General Psychiatry. 1989;46(3):225–230. doi: 10.1001/archpsyc.1989.01810030031004. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Saladin M, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug and Alcohol Dependence. 2002;65(2):115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences (Rev ed.) Hillsdale, NJ England: Lawrence Erlbaum Associates, Inc.; 1977. [Google Scholar]
- Creamer M, Burgess P, McFarlane AC. Post-traumatic stress disorder: Findings from the Australian National Survey of Mental Health and Well-Being. Psychological Medicine. 2001;31(7):1237–1247. doi: 10.1017/s0033291701004287. [DOI] [PubMed] [Google Scholar]
- Dass-Brailsford P, Myrick AC. Psychological trauma and substance abuse: The need for an integrated approach. Trauma, Violence, and Abuse. 2010;11(4):202–213. doi: 10.1177/1524838010381252. [DOI] [PubMed] [Google Scholar]
- Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S, Goodwin L. A systematic review of the comorbidity between PTSD and alcohol misuse. Social Psychiatry and Psychiatric Epidemiology. 2014 doi: 10.1007/s00127-014-0855-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods. 2009;14(1):43. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition with psychotic screen (SCID-I/P W/ PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Oslin D, Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. The Journal of the American Medical Association. 2013;310(5):488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Forbes D, Creamer M, Bisson JI, Cohen JA, Crow BE, Foa EB, Ursano RJ. A guide to guidelines for the treatment of PTSD and related conditions. Journal of Traumatic Stress. 2010;23(5):537–552. doi: 10.1002/jts.20565. [DOI] [PubMed] [Google Scholar]
- Friedman M. PTSD: Pharmacotherapeutic approaches, FOCUS: The Journal of Lifelong Learning in Psychiatry. 2013;11(3):15–320. [Google Scholar]
- Gorelick DA, Paredes A. Effect of fluoxetine on alcohol consumption in male alcoholics. Alcoholism: Clinical and Experimental Research. 1992;16(2):261–265. doi: 10.1111/j.1530-0277.1992.tb01373.x. [DOI] [PubMed] [Google Scholar]
- Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold PTSD in primary care: Prevalence, psychiatric disorders, healthcare use, and functional status. The Journal of Nervous and Mental Disease. 2005;193(10):658–664. doi: 10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- Hallgren KA, Witkiewitz K. Missing data in alcohol clinical trials: a comparison of methods. Alcoholism: Clinical and Experimental Research. 2013;37(12):2152–2160. doi: 10.1111/acer.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Jiang H, Campbell AN, Hu MC, Miele GM, Cohen LR, Nunes EV. Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA's Clinical Trials Network. The American Journal of Psychiatry. 2010;167(1):95–101. doi: 10.1176/appi.ajp.2009.09091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Wells EA, Jiang H, Suarez-Morales L, Campbell AN, Cohen LR, Nunes EV. Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. Journal of Consulting Clinical Psychology. 2009;77(4):607–619. doi: 10.1037/a0016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs JD, Kushner MG, Lee SS, Reardon SM, Maurer EW. Meta-analysis of supplemental treatment for depressive and anxiety disorders in patients being treated for alcohol dependence. The American Journal on Addictions. 2011;20(4):319–329. doi: 10.1111/j.1521-0391.2011.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD) The International Journal of Neuropsychopharmacology. 2012;15(6):825–840. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Garbutt JC. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A systematic review and meta-analysis. JAMA: The Journal of the American Medical Association. 2014;311(18):1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Amin H, Modesto-Lowe V, Oncken C. Pharmacologic treatments for drug and alcohol dependence. Psychiatric Clinics of North America. 1999;22(2):401–423. doi: 10.1016/s0193-953x(05)70084-8. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H. Posttreatment outcomes in a double-blind, randomized trial of sertraline for alcohol dependence. Alcoholism: Clinical and Experimental Research. 2012;36(4):739–744. doi: 10.1111/j.1530-0277.2011.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias AJ, Oncken C. A double-blind, randomized trial of sertraline for alcohol dependence: Moderation by age and 5-hydroxytryptamine transporter-linked promoter region genotype. Journal of Clinical Psychopharmacology. 2011;31(1):22–30. doi: 10.1097/JCP.0b013e31820465fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcoholism: Clinical and Experimental Research. 1996;20(9):1534–1541. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Burleson JA, Korner P, Del Boca FK, Bohn MJ, Brown J, Liebowitz N. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. American Journal of Psychiatry. 1995;152(3):391–397. doi: 10.1176/ajp.152.3.391. [DOI] [PubMed] [Google Scholar]
- Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83(404):1198–1202. [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, Back SE. Posttraumatic stress disorder and co-occurring substance use disorders: Advances in assessment and treatment. Clinical Psychology: Science and Practice. 2012;9(3):283–304. doi: 10.1111/cpsp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Back SE, Brady KT, Baker AL, Hopwood S, Ewer PL. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: A randomized controlled trial. JAMA: The Journal of the American Medical Association. 2012;308(7):690–699. doi: 10.1001/jama.2012.9071. [DOI] [PubMed] [Google Scholar]
- Morgan-Lopez AA, Saavedra LM, Hien DA, Campbell AN, Wu E, Ruglass L, Bainter SC. Indirect effects of seeking safety on substance use outcomes: Overall and attendance class-specific effects. American Journal on Addictions. 2014;23(3):218–225. doi: 10.1111/j.1521-0391.2014.12100.x. [DOI] [PubMed] [Google Scholar]
- Najavits LM. Seeking safety: A treatment manual for PTSD and substance abuse. New York: Guilford Press; 2002. [Google Scholar]
- Najavits LM. Seeking Safety Session Format Checklist. Belmont, MA: Unpublished manuscript, McLean Hospital; 2003. [Google Scholar]
- Najavits LM, Hien DA. Helping vulnerable populations: A comprehensive review of the treatment outcome literature on substance use disorders and PTSD. Journal of Clinical Psychology: In Session. 2013;69(5):433–479. doi: 10.1002/jclp.21980. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Weiss RD, Liese BS. Group cognitive-behavioral therapy for women with PTSD and substance use disorder. Journal of Substance Abuse Treatment. 1996;13(1):13–22. doi: 10.1016/0740-5472(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Kadlec KE, Sanhueza P, Woodley-Remus D, Sellers EM. Fluoxetine differentially alters alcohol intake and other consummatory behaviors in problem drinkers. Clinical Pharmacology & Therapeutics. 1990;47(4):490–498. doi: 10.1038/clpt.1990.62. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Sellers EM, Sullivan JT, Woodley DV, Kadlec K, Sykora K. The serotonin uptake inhibitor citalopram attenuates ethanol intake. Clinical Pharmacology & Therapeutics. 1987;41(3):266–274. doi: 10.1038/clpt.1987.27. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Sullivan JT, Kadlec KE, Woodley-Remus DV, Kennedy G, Sellers EM. Differential effects of viqualine on alcohol intake and other consummatory behaviors. Clinical Pharmacology & Therapeutics. 1989;46(3):301–309. doi: 10.1038/clpt.1989.142. [DOI] [PubMed] [Google Scholar]
- NIAAA. Moderate & Binge Drinking. [Retrieved October 20, 2013];2013 from http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Norman SB, Myers US, Wilkins KC, Goldsmith AA, Hristova V, Huang Z, Robinson SK. Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology. 2012;62(2):542–551. doi: 10.1016/j.neuropharm.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette PC, Ahrens C, Moos RH, Finney JW. Posttraumatic stress disorder in substance abuse patients: Relationship to 1-year posttreatment outcomes. Psychology of Addictive Behaviors. 1997;11(1):34–47. [Google Scholar]
- Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, Krystal JH. Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology. 2012;37(4):996–1004. doi: 10.1038/npp.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biological Psychiatry. 2004;56(10):785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: Interactive effects of medication and alcoholic subtype. Alcoholism: Clinical and Experimental Research. 2000;24(7):1041–1049. [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity. Project MATCH posttreatment drinking outcomes. Journal on Studies of Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Ralevski E, Olivera-Figueroa LA, Petrakis I. PTSD and comorbid AUD: A review of pharmacological and alternative treatment options. Journal of Substance Abuse and Rehabilitation. 2014;7(5):25–36. doi: 10.2147/SAR.S37399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roache JD, Wang Y, Ait-Daoud N, Johnson BA. Prediction of serotonergic treatment efficacy using age of onset and Type A/B typologies of alcoholism. Alcoholism: Clinical and Experimental Research. 2008;32(8):1502–1512. doi: 10.1111/j.1530-0277.2008.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannibale C, Teesson M, Creamer M, Sitharthan T, Bryant RA, Sutherland K, Peek-O’Leary M. Randomized controlled trial of cognitive behaviour therapy for comorbid post-traumatic stress disorder and alcohol use disorders. Addiction. 2013;108(8):1397–1410. doi: 10.1111/add.12167. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Neria Y, Pavlicova M, Hembree E, Sun EJ, Amsel L, Marshall RD. Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: A randomized controlled trial. The American Journal of Psychiatry. 2012;169(1):80–88. doi: 10.1176/appi.ajp.2011.11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin S. Statistical analysis of epidemiologic data. 2nd ed. New York: Oxford University Press; 1996. [Google Scholar]
- Smith JP, Randall CL. Anxiety and alcohol use disorders: Comorbidity and treatment considerations. Alcohol Research & Health. 2012;34(4):414–431. [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ US: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Torchalla I, Nosen L, Rostam H, Allen P. Integrated treatment programs for individuals with concurrent substance use disorders and trauma experiences: A systematic review and meta-analysis. Journal of Substance Abuse Treatment. 2012;42(1):65–77. doi: 10.1016/j.jsat.2011.09.001. [DOI] [PubMed] [Google Scholar]
- van Dam D, Vedel E, Ehring T, Emmelkamp PMG. Psychological treatments for concurrent posttraumatic stress disorder and substance use disorder: A systematic review. Clinical Psychology Review. 2012;32(3):202–214. doi: 10.1016/j.cpr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Back SE, Verduin ML, Brady KT. Triggers for cocaine and alcohol use in the presence and absence of posttraumatic stress disorder. Addictive Behaviors. 2007;32(3):634–639. doi: 10.1016/j.addbeh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-Administered PTSD Scale: A review of the first ten years of research. Depression and anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]