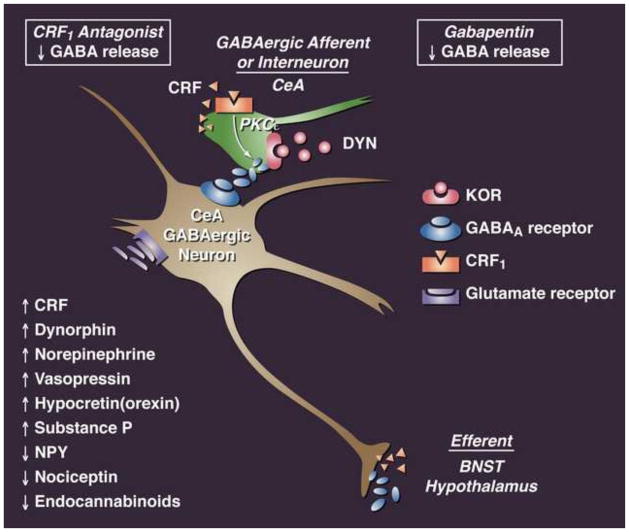
Fig. 2.
Cellular neuroadaptive mechanisms in the central nucleus of the amygdala in drug addiction. Simplified schematic of rodent central nucleus of the amygdala circuitry and hypothetical sites of ethanol and CRF action on GABAergic synapses. Most neurons in the CeA are GABAergic inhibitory projection neurons or interneurons that contain CRF or other neuropeptides as cotransmitters. (Upper synapse) Ethanol may enhance the release of GABA (filled ellipsoids) from GABAergic afferents or interneurons either via release from the same terminal as CRF (gray triangles), which then acts on CRF1 receptors on the terminal to elicit (black arrow) release of more GABA via a PKCε-mediated mechanism, or direct activation of CRF1 receptors to elicit the release of more GABA (Bajo et al., 2008). CRF1 antagonists and the drug gabapentin decrease presynaptic GABA release in dependent animals (Roberto et al., 2008, 2010). κ-Opioid antagonists have similar effects as CRF1 antagonists in rats that present an escalation in cocaine intake (Kallupi et al., 2013). Thus, CRF, dynorphin, and ethanol augment the inhibition of CeA projection interneurons (co-containing CRF, opioids, or NPY), leading to the excitation of downstream (e.g., BNST) neurons through disinhibition. The activation of presynaptic cannabinoid CB1 or NPY receptors (data not shown) may reduce GABA release onto CeA inhibitory projection neurons, increasing their excitability and release of GABA onto downstream targets, such as in the BNST. CRF, corticotropin-releasing factor; GABA, γ-aminobutyric acid; CeA, central nucleus of the amygdala; DYN, dynorphi; KOR, κ opioid receptor; NPY, neuropeptide Y; BNST, bed nucleus of the stria terminalis.