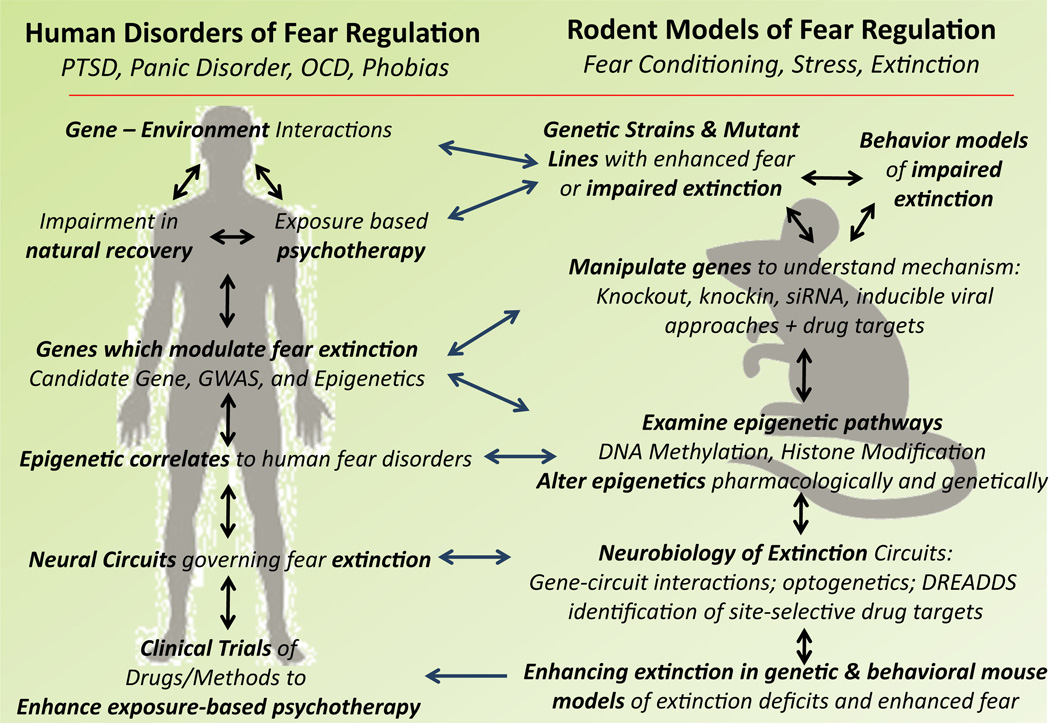
Fig. 10.
Strategy for translating research from humans to mice and back, focusing on human disorders of fear dysregulation. Rodent models of enhanced fear and impaired extinction (induced by genetic or environmental manipulations) closely resemble human symptomatology, particularly the fear dysregulation that occurs in PTSD, panic, and phobic disorders. Using these models increases the chances of identifying candidate genes for human anxiety disorders by reducing the problems of genetic heterogeneity and a variable environment. It is also important to study the involvement of these candidate genes in patients, as well as using unbiased genome-wide association studies and genome-wide epigenetic approaches to identify previously unknown genetic pathways contributing to risk in humans. Subsequently, elucidating the function of the identified gene and its epigenetic regulation using rodent models as well as the human population increases our knowledge of the neurobiology of fear disorders, which, at the same time, is necessary to improve the models. It is also necessary to use rodent models to test the ability to pharmacologically enhance extinction of fear and diminish fear expression prior to clinical test phases. Arrows demonstrate how the different levels of understanding, from genetics to epigenetics to neural circuits, can inform each other, as well as how they can be informed across species due to the high level of convergence of shared fear-related processing across mammals.