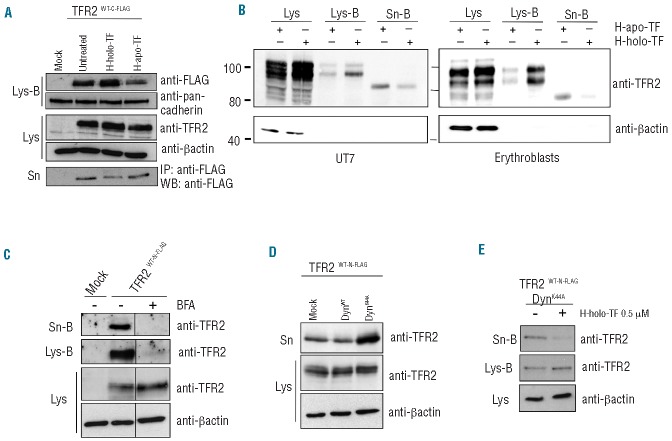
Figure 2.
sTFR2 is cleaved from the plasma membrane. (A) HeLa cells were transiently transfected with empty vector (mock) and TFR2WT-C-FLAG coding vector. Eighteen hours after transfection the media were replaced with serum-free media to which human holo-transferrin (H-holo-TF, 25 μM) or human apo-transferrin (H-apo-TF, 25 μM) was added or not. After 24 h media were collected and concentrated, cells were biotinylated to label membrane protein then lysed. sTFR2 was pulled down using anti-FLAG Sepharose-beads. Cellular lysates were precipitated with streptavidin to analyze proteins originating from the cell surface. Total lysates (Lys), biotinylated samples (Lys-B) and concentrated media (Sn) were analyzed by western blot using anti-TFR2 (Abcam), anti-FLAG antibody. Anti-pan-cadherin, which recognizes a plasma membrane protein, was used to normalize plasma membrane biotinylation and streptavidin pull-down. (B) An erythroid cell line UT7 (left panel) and erythro blasts (right panel) were incubated for 24 h in the presence of 25 μM human apo-TF or holo-TF. After biotinylation of cell surface proteins, cell lysates (Lys-B) and supernatant (Sn-B) were precipitated using neutravidin agarose beads. Western blots were performed using an anti-TFR2 antibody (Santa Cruz). Scales refer to relative molecular mass in kilo Daltons. (C) HeLa cells transiently transfected with empty vector (mock) and wild-type TFR2 coding vector (TFR2WT-N-FLAG) were treated with brefeldin-A (BFA, 100 ng/mL) to block intracellular trafficking and protein export to the plasma membrane. After 18 h cells were incubated with biotin to label membrane proteins and than re-incubated with BFA. Twenty-four hours later media were collected and concentrated, cells lysed (Lys) and both media (Sn-B) and lysates (Lys-B) were subjected to streptavidin pull-down and western blot analysis using an anti-TFR2 antibody (Abcam). (D) The cell culture media and the total lysates of HeLa cells, transiently transfected with wild type TFR2 (TFR2WT-N-FLAG) in combination with DynWT and DynK44A expressing vectors, were analyzed by western blot using anti-TFR2 (Abcam). (E) HeLa cells were transiently transfected with wild-type TFR2 (TFR2WT-N-FLAG) and DynK44A expressing vectors. After 18 h cells were incubated with biotin to label membrane proteins and were incubated in serum-free media, treated or not with H-holo-TF (0.5 μM). After 24 h media were collected and concentrated and cells were lysed. Both media (Sn-B) and lysates (Lys-B) were precipitated with streptavidin to analyze by western blot proteins originating from the cell surface using an anti-TFR2 antibody (Abcam). Loading was estimated with anti-β-actin antibody. The results were confirmed several times in different experiments. Western blots of representative experiments are shown.