Figure 2.
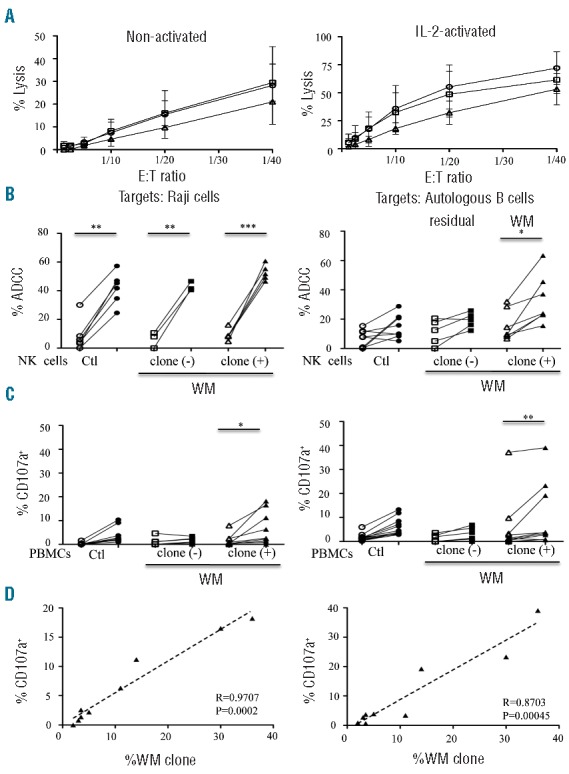
Cytolytic activities of natural killer (NK) cells from Waldenström macroglobulinemia (WM) patients with or without circulating B-cell clones. (A) Direct lysis of non-activated (left panel) and IL2-activated (right panel) NK cells from healthy donors (Ctl; circles; n=6) and WM patients without [clone (-); squares; n=4] or with [clone (+); triangles; n=3] circulating B-cell clones. Cytotoxicity was measured using standard 51Cr release-assays against K562 target cells at different effector:target cell (E:T) ratios. Mean percentage values±s.d. (vertical bars) are shown. (B) Comparison of NK cell ADCC in the presence of rituximab (open symbols) or ublituximab (closed symbols) against Raji cells at 1 ng/mL of anti-CD20 (left panel) or against purified peripheral blood autologous B cells at 100 ng/mL of anti-CD20 (right panel). Assays were performed with purified NK cells from healthy donors (Ctl; circles) and from WM patients without [clone (−); squares] or with [clone (+); triangles] circulating B-cell clones (WM clone) (E:T ratio at 1:10). (C) Degranulation response determined using CD107a expression on CD3‒CD56+ NK cells in the presence of 10 ng/mL (left panel) or 1,000 ng/mL (right panel) of rituximab (open symbols) or ublituximab (closed symbols). Assays were performed on fresh peripheral blood mononuclear cells (PBMC) from healthy donors (Ctl; circles) or WM patients without [clone (−); squares] or with [clone (+); triangles] circulating B-cell clones (WM clone). P values were calculated with the Wilcoxon rank sum test and refer to the comparison between rituximab and ublituximab; *P<0.05, **P<0.001, ***P<0.0001. (D) Linear regression between the percentage of CD107a expression on CD3‒CD56+ NK cells and the percentage of circulating B-cell clones (WM clone) after treatment with 10 ng/mL (left panel) or 1000 ng/mL (right panel) of ublituximab. All data relative to ADCC and CD107a expression are presented after subtraction of the values obtained in the absence of any antibody.
