Figure 1.
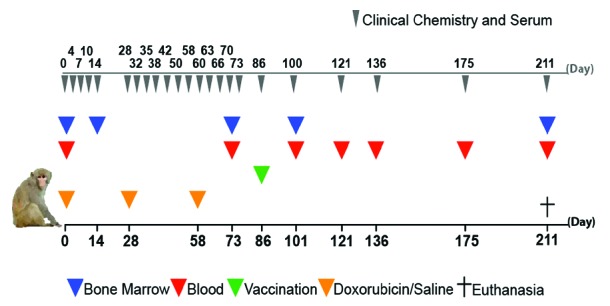
Ten measles-immune rhesus macaques, 11 to 15 years old, were included in the study. The chemotherapy group (n=5) was treated with 30 mg/m2, 50 mg/m2 and 75 mg/m2 of doxorubicin diluted with 0.9% sodium chloride, infused for 60 min (orange triangles) and the control group (n=5) received saline (orange triangles) under identical conditions. Primary (rubella and tetanus) and secondary (measles) vaccine responses were evaluated after vaccination of all animals with 0.5 mL subcutaneous M.M.RVaxPro© (Sanofi Pasteur MSD) and 0.5 mL intramuscular Tetanus Toxoid Vaccine (Netherlands Vaccine Institute) on day 86. Blood (14 mL, red triangles) and bone marrow aspirate (blue triangles) were sampled for isolation of peripheral-blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs). For assessment of clinical chemistry, 1 mL blood (gray triangles on top) was taken and serum stored. Due to technical issues study day 0, there was not enough BM sample to perform all analyses in some animals. On the basis of unaltered neutrophil counts (Online Supplementary Table S1), 1 BM sample from the treated group and 3 BM samples from the control group which represent baseline values are from study day 14. At the end of the study the animals were euthanized by an overdose of pentobarbital. The study was approved by the Institutional Animal Care and Use Committee (IACUC) of BPRC.
