Abstract
Inorganic polyphosphate is a universally conserved biopolymer, whose association with oxidative stress resistance has been documented in many species, but whose mode of action has been poorly understood. Here we review the recent discovery that polyphosphate functions as a protein-protective chaperone, examine the mechanisms by which polyphosphate-metal ion interactions reduce oxidative stress, and summarize polyphosphate’s roles in regulating general stress response pathways. Given the simple chemical structure and ancient pedigree of polyphosphate, these diverse mechanisms are likely to be broadly relevant in many organisms, from bacteria to mammalian cells.
INTRODUCTION
Oxidative stress is an inevitable consequence of aerobic life. Reduction of molecular oxygen generates a series of reactive oxygen species (ROS), including superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•). ROS are capable of damaging proteins, DNA, lipids, and other cellular components, and exert powerful, and under some conditions, potentially lethal stress on bacterial cells [1,2]. The types of damage caused by each of these species and the mechanisms by which bacteria defend themselves have recently been reviewed [3]. Many bacterial defenses against oxidative stress are based on changes in gene expression, upregulating the cellular concentration of enzymes that scavenge superoxide and hydrogen peroxide or repair oxidative damage to DNA and iron-sulfur clusters in proteins. In addition, however, several post-translational response mechanisms have been identified, involving the redox-regulated adjustment of cellular metabolism to oxidative stress conditions, the activation of specific molecular chaperones and the accumulation of inorganic polyphosphate (polyP). Together, these systems provide powerful protection against oxidative stress conditions in bacteria.
Inorganic polyphosphate is a polymer of phosphoanhydride-linked phosphate residues, found as chains up to 1000 residues long in cells from all three domains of life [4]. Despite its universal nature, the roles of polyP in cellular metabolism are only beginning to be understood. PolyP has long been known to affect the ability of a variety of prokaryotic and eukaryotic cells to resist oxidative stress. Arthur Kornberg and coworkers showed in 1992 that disruption of the Escherichia coli ppk gene that encodes polyP kinase (PPK), the enzyme catalyzing the reversible synthesis of polyP from ATP [5], resulted in increased sensitivity towards multiple stressors, including heat, starvation, and H2O2 [6]. Since then, ppk mutants of many species of bacteria have been shown to be sensitive to ROS treatment (for examples, see [4,7–10]). However, until very recently, the molecular mechanisms by which polyP protects cells against oxidative stress have been uncertain. An increased understanding of the molecular mechanisms by which polyP acts is likely to have broad implications, especially considering recent developments showing that polyP is a signal molecule controlling inflammation in mammals [11] (a process that involves production of considerable ROS) and that bacterial polyP can modulate host inflammatory responses [12,13].
In this review, we discuss exciting advances of the past three years that have demonstrated mechanistic details of multiple pathways by which polyP both directly and indirectly protects bacteria from oxidative stress. These range from direct protein-stabilizing chaperone activity, to interactions with redox-active metals, to regulatory roles in controlling stress response pathways.
POLYPHOSPHATE IS A PROTEIN-PROTECTING CHAPERONE
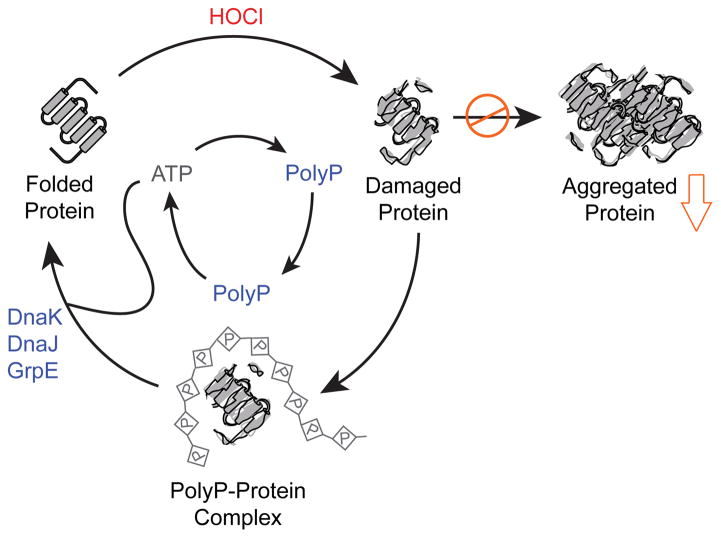
Hypochlorous acid (HOCl), the active ingredient of household bleach, is an extremely potent microbicidal oxidant whose effects on bacterial cells we have recently reviewed [14]. HOCl is notable among ROS for causing extremely rapid protein damage by oxidizing cysteine, methionine, histidine, and other amino acids, leading to unfolding and aggregation of oxidized proteins [15]. A recent study of the E. coli response to HOCl showed that treatment of bacteria with sublethal doses of HOCl caused the rapid accumulation of polyP, and that E. coli mutants lacking PPK were exquisitely sensitive to HOCl treatment [8]. These observations led to the question of how polyP was protecting cells against HOCl, and to the unexpected discovery that polyP interacts directly with unfolding proteins and prevents their aggregation, acting as an inorganic chaperone (Figure 1). Physiological concentrations of polyP were able to efficiently prevent protein aggregation both in vivo and in vitro, with longer chains of polyP exerting a stronger protective effect than shorter chains. PolyP was able to protect a broad spectrum of proteins from aggregation, suggesting that this chaperone function is very likely to be important in any cell that accumulates polyP. Moreover, and in true protein chaperone-like fashion, polyP maintained proteins in a refolding competent conformation, handing its clients over for successful refolding by the DnaK/DnaJ/GrpE chaperone machinery. PolyP synthesis is directly regulated by HOCl via reversible oxidative inactivation of the polyP-degrading exopolyphosphatase PPX [8], allowing it to bypass time-consuming transcription/translation processes. In addition, PolyP has several other distinct advantages over more conventional protein chaperones under severe oxidative stress conditions. Unlike proteins, polyP does not react with HOCl or other ROS [15]. It does not require ATP for its function and in fact, upon release of stress, polyP can be efficiently converted back into ATP by PPK [5], which can then be used by ATP-dependent chaperones to promote protein refolding [8].
Figure 1.
Polyphosphate acts as a chaperone to prevent aggregation of oxidatively damaged proteins. Proteins damaged by oxidation, especially by the strong oxidant HOCl, are prone to cytotoxic aggregation. PolyP, generated from ATP under oxidative stress conditions, forms stable complexes with unfolding proteins, keeping them soluble and competent to be refolded. Upon relief of stress, polyP can be reconverted to ATP, which can then be used by ATP-dependent chaperones (e.g. DnaK, DnaJ, and GrpE) to refold polyP-protected proteins. Modified with permission from [8]. Abbreviations: HOCl, hypochlorous acid; ATP, adenosine triphosphate; polyP, inorganic polyphosphate.
Many questions about the chaperone function of polyP remain unanswered. How does polyP interact with unfolding proteins to prevent their aggregation? To what extent does loss of polyP chaperone function explain the pleitotropic phenotypes of ppk mutants in bacteria, which are commonly sensitive to multiple stresses and defective in motility, biofilm formation, and virulence [4]? What characterizes the client proteins of polyP, and is there an overlap between polyP substrates and the clients of other molecular chaperones?
MANGANESE-PHOSPHATE DETOXIFIES SUPEROXIDE
Aerobic organisms contain multiple superoxide dismutase enzymes which scavenge O2−, preventing inactivation of essential iron-sulfur cluster and mononuclear iron-containing enzymes [3]. However, some aerotolerant anaerobes, such as the lactic acid bacterium Lactobacillus plantarum, do not contain superoxide dismutase [16,17]. As reviewed recently [17], these organisms compensate for the loss of enzymatic superoxide dismutase by accumulating high concentrations of Mn2+ ions which, when coordinated by a variety of organic and inorganic metabolites, are able to detoxify O2−. In L. plantarum, non-enzymatic superoxide dismutase activity was originally (in 1982) associated with Mn2+-polyP complexes [16], indicating that polyP might play a role in this process. The more recent finding that Saccharomyces cerevisiae mutants lacking superoxide dismutase can be rescued by addition of manganese, but mutants lacking both superoxide dismutase and the polyP polymerase Vtc4 cannot [18,19], further reinforced this association of polyP with Mn2+-dependent antioxidant activity.
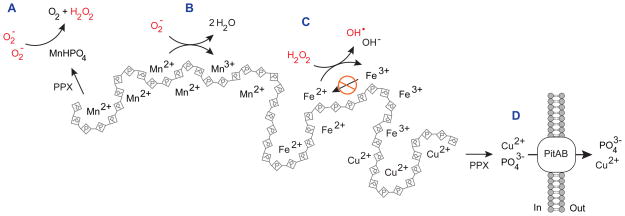
Until very recently, the physiologically relevant ligand(s) and chemical mechanism of Mn2+-dependent superoxide scavenging were controversial. In 2012, however, Valentine and coworkers provided experimental proof that manganous phosphate (MnHPO4) and manganous carbonate (MnCO3) catalyze superoxide dismutation at rates competitive with enzymatic superoxide dismutase [20]. In contrast, they found that manganous pyrophosphate (MnP2O72−) reacted only stoichiometrically with O2−, resulting in non-catalytic detoxification of O2− and oxidation of Mn2+ to Mn3+. A dual, catalytic and non-catalytic model would explain the mechanism(s) behind the observed role of polyP in managnese-dependent detoxification of O2− (Figure 2). Since polyP efficiently coordinates divalent cations like Mn2+ [21], it inevitably stabilizes the cellular Mn2+ pool. Hydrolysis of Mn2+-polyP by PPX will then generate MnHPO4, which will rapidly and catalytically detoxify O2− (Figure 2A). Alternatively, and in analogy to the reaction observed with MnP2O72−, Mn2+-polyP may non-catalytically detoxify O2−, resulting in accumulation of Mn3+-polyP (Figure 2B). In either case, the cell is efficiently protected from ROS without the need for superoxide dismutase. To test this model, however, future experiments are necessary that examine the role of PPX in O2− detoxification and measure levels of Mn3+ in O2−-stressed bacteria.
Figure 2.
Polyphosphate-metal complexes play multiple roles in oxidative stress resistance. (A) MnHPO4, likely to be generated by PPX digestion of manganese-polyP complexes, catalyzes dismutation of superoxide to O2 and H2O2. (B) Mn2+ ions in complex with polyP can non-catalytically quench superoxide, yielding Mn3+ and H2O. (C) Complex formation between polyP and redox active metals (e.g. Fe2+, Cu2+) reduces the yield of hydroxyl radicals by slowing regeneration of the Fenton-reactive Fe2+. (D) PolyP facilitates export of Cu2+ via a process requiring PPX and the metal-phosphate symporters PitA and PitB. Abbreviations: PPX, exopolyphosphatase; H2O2, hydrogen peroxide; OH•, hydroxyl radical; O2−, superoxide.
POLYPHOSPHATE DEFENDS AGAINST THE FENTON REACTION
Reaction of H2O2 or HOCl with redox-active metal ions like Fe2+ or Cu2+ generates the highly reactive and toxic hydroxyl radical (OH•) via the Fenton reaction [3]. At least two distinct mechanisms explain how polyP might reduce the cytotoxic effects of the Fenton reaction. In vitro studies have been shown that PolyP dramatically reduces the OH• yield of the Fenton reaction (Figure 2C) [22], despite the fact that chelation of Fe2+ by polyP accelerates the rate of the Fenton reaction by several orders of magnitude [23]. The explanation for this apparent contradiction appears to lie in the ability of polyP to stabilize the Fe3+ intermediate, thereby inhibiting the regeneration of Fe2+, which is necessary for additional cycles of OH• generation [23]. However, additional work is needed to validate this model in vivo. In addition, polyP has been shown to facilitate export of Cu2+ from the cell (Figure 2D). The details of this mechanism have recently been elucidated in E. coli, where Cu2+ tolerance was shown to depend on polyP synthesis by PPK, polyP degradation by PPX, and the metal-phosphate symporters PitA or PitB [24], supporting a model in which Cu2+ is first chelated by polyP to reduce its toxicity, followed by PPX cleavage of PO43− from polyP and co-export of the resulting PO43− and Cu2+ via the Pit system. This model is consistent with earlier work on Cu2+-resistance in Sulfolobus metallicus [25] and both Cd2+ and Hg2+ resistance in E. coli [26,27]. These studies indicate that polyP’s involvement in metal export is conserved very broadly among prokaryotes and that it is not limited to the Fenton reaction-catalyzing metal Cu2+ but applies also to other metals like Cd2+ or Hg2+, which are known to oxidize cysteine residues [28].
POLYPHOSPHATE IS REQUIRED FOR FORMATION OF PERSISTER CELLS
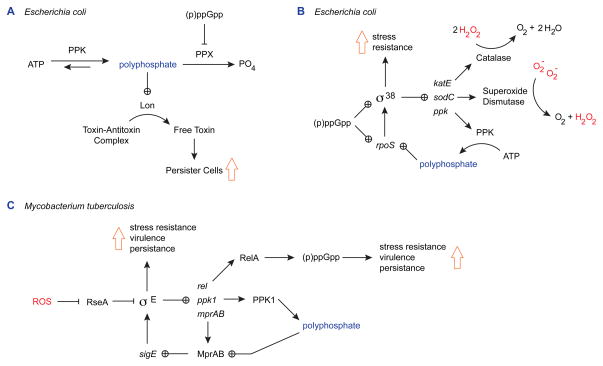
Persister cells are members of a bacterial population that stochastically enter a dormant state, in which they become highly stress-resistant (reviewed recently in [29]). Although this phenomenon has been studied largely in the context of antibiotic resistance, formation of persister cells is also stimulated by ROS [30]. PolyP has recently been shown to play a central role in the molecular mechanism by which bacteria enter this stress-tolerant state (Figure 3A) [31]. Persister cells arise due to stochastic accumulation of high levels of the alarmone (p)ppGpp in a subpopulation of cells. Since(p)ppGpp has been shown to inactivate the polyP-degrading enzyme PPX [32], polyP accumulates. PolyP, in turn, is thought to interact with the Lon protease, stimulating digestion of a subset of cellular proteins [33]. These proteins include the antitoxin modules of Type II toxin-antitoxin systems [31]. The free toxins (e.g. HipA, RelE, MazF, etc.) then go on to inhibit cell metabolism at a variety of levels, including transcription, translation, and DNA replication, with the result that affected cells enter a slow-growing persister state [29]. Populations of mutants lacking PPK form dramatically lower levels of persister cells, reinforcing the importance of polyP in control of this stress response mechanism [31].
Figure 3.
Polyphosphate regulates general stress response networks. (A) In E. coli, the stress alarmone (p)ppGpp inhibits PPX, stimulating accumulation of polyP. PolyP activates Lon protease, which then degrades the antitoxin components of type II toxin-antitoxin systems. Accumulation of free toxin reduces growth rate and leads to an increase in formation of broadly stress-resistant persister cells. (B) In E. coli, the general stress response regulon controlled by the sigma factor σ38 includes genes encoding PPK, catalase, and superoxide dismutase. PolyP activates expression of the rpoS gene encoding σ38. (C) In M. tuberculosis, polyP is required for MprAB-dependent expression of the stress response sigma factor σE, which controls polyP and (p)ppGpp biosynthesis and is required for stress resistance, virulence, and persistence in macrophages. Oxidative stress (e.g. H2O2) leads to activation of σE by inactivating the σE anti-sigma factor RseA. Abbreviations: ATP, adenosine triphosphate; (p)ppGpp, guanosine penta-or tetra-phosphate; PPK and PPK1, polyphosphate kinase; PPX, exopolyphosphatase; H2O2, hydrogen peroxide; O2−, superoxide.
POLYPHOSPHATE IS INVOLVED IN REGULATION OF GENERAL STRESS RESPONSE NETWORKS
It has been known for some time that polyP plays a role in regulation of the σ38-dependent general stress response system of E. coli (Figure 3B) [34–37]. This conclusion was based on the observation that polyP is required for efficient transcription of rpoS, the gene encoding σ38 [36], but the mechanism by which this occurs remains mysterious, and is an intriguing question for future research. The σ38 regulon includes many genes encoding enzymes important for ROS resistance, including katE (encoding catalase), sodC (encoding superoxide dismutase), and ppk itself [38]. A mutant lacking σ38 does not accumulate polyP in response to osmotic stress or nitrogen starvation [34], indicating the presence of a feedback loop between polyP and rpoS expression in E. coli. Evidence for a similar role of polyP in control of rpoS expression in Pseudomonas putida has recently been published [10].
More recently, studies in Mycobacterium spp. have also demonstrated a role for polyP in the regulation of sigma factor σE, which controls a stress-responsive regulon required for virulence, persistence in macrophages, and resistance to a variety of stresses, including oxidative stress and phosphate starvation [39,40]. Many of the components of this regulatory loop and the resulting stress-resistance phenotypes are conserved among even distantly related bacteria (Figure 3C). It has been shown that the correct control of this regulon depends on the ability of M. tuberculosis to synthesize polyP and the two-component transcriptional regulatory system MprAB, which activates sigE expression in response to polyP [41,42]. ROS-dependent activation of σE activity is mediated by the redox-sensitive anti-sigma factor RseA [43], and leads to the expression of rel (encoding the (p)ppGpp synthase RelA), ppk1 (encoding the M. tuberculosis polyP kinase PPK1), and mprAB. These gene products help to increase the amount of polyP in the cell [40,41] and control the circuit.
While evidence from a number of bacteria supports the involvement of polyP in regulation of general stress responses, the precise details appear to differ somewhat among species. Common themes include regulatory loops in which polyP is required for expression of sigma factors that drive ppk gene expression, coordinated roles for polyP and the alarmone (p)ppGpp, and a central role for polyP in regulatory circuits controlling stress-resistance enzymes, including those that detoxify ROS (Figure 3). Determining the exact mechanism by which polyP regulates gene expression and the extent of conservation of these pathways in different bacteria represent interesting avenues for future research.
CONCLUSIONS
Exciting progress has been made in the last three years in the understanding of how polyP contributes to oxidative stress resistance, and has highlighted the profoundly multifunctional nature of this ancient biopolymer. Polyphosphate has multiple functions that increase bacterial oxidative stress resistance. As a chaperone, polyP prevents aggregation of damaged proteins. As a metal chelator, polyP facilitates detoxification of superoxide, reduces the free radical yield of the Fenton reaction, and promotes export of toxic redox-active metals. PolyP is also intimately involved in regulation of general stress response pathways in different bacteria, including those that lead to stress-resistant persister cells and to expression of a variety of antioxidant enzymes. While this multifunctionality complicates the interpretation of experiments, requiring careful controls to distinguish which mechanism(s) are playing the most important role(s) under specific conditions, it goes a long way towards explaining the diverse and pleiotropic phenotypes associated with polyP.
HIGHLIGHTS.
Polyphosphate protects bacteria from oxidative stress by multiple mechanisms.
Polyphosphate is a chaperone that prevents aggregation of oxidized proteins.
Polyphosphate-metal complexes lower levels of ROS and redox-active metals in cells.
Polyphosphate is a key regulator of persister cell formation.
Polyphosphate is involved in regulation of general stress response networks.
Acknowledgments
This work was supported by grants F32GM096613 and R01GM065318 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael J. Gray, Email: mikegray@umich.edu.
Ursula Jakob, Email: ujakob@umich.edu.
References
- 1.Zhao X, Drlica K. Reactive oxygen species and the bacterial response to lethal stress. Curr Opin Microbiol. 2014;21C:1–6. doi: 10.1016/j.mib.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imlay JA. Diagnosing oxidative stress: not as easy as you might think. Curr Opin Microbiol. 2015 doi: 10.1016/j.mib.2015.01.004. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao NN, Gomez-Garcia MR, Kornberg A. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem. 2009;78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 5.Ahn K, Kornberg A. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J Biol Chem. 1990;265:11734–11739. [PubMed] [Google Scholar]
- 6.Akiyama M, Crooke E, Kornberg A. The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. J Biol Chem. 1992;267:22556–22561. [PubMed] [Google Scholar]
- 7.Alcantara C, Blasco A, Zuniga M, Monedero V. Polyphosphate in Lactobacillus: accumulation and involvement in resistance against stress. Appl Environ Microbiol. 2013 doi: 10.1128/AEM.03997-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Gray MJ, Wholey W-Y, Wagner NO, Cremers CM, Mueller-Schickert A, Hock NT, Krieger AG, Smith EM, Bender RA, Bardwell JCA, et al. Polyphosphate is a primordial chaperone. Mol Cell. 2014;53:1–11. doi: 10.1016/j.molcel.2014.01.012. Demonstration that polyP is a molecular chaperone that prevents protein aggregation in E. coli treated with HOCl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahid IK, Silva AJ, Benitez JA. Polyphosphate stores enhance the ability of Vibrio cholerae to overcome environmental stresses in a low-phosphate environment. Appl Environ Microbiol. 2006;72:7043–7049. doi: 10.1128/AEM.00924-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikel PI, Chavarria M, Martinez-Garcia E, Taylor AC, de Lorenzo V. Accumulation of inorganic polyphosphate enables stress endurance and catalytic vigour in Pseudomonas putida KT2440. Microb Cell Fact. 2013;12:50. doi: 10.1186/1475-2859-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SA, Morrissey JH. Polyphosphate: a new player in the field of hemostasis. Curr Opin Hematol. 2014;21:388–394. doi: 10.1097/MOH.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Segawa S, Fujiya M, Konishi H, Ueno N, Kobayashi N, Shigyo T, Kohgo Y. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS One. 2011;6:e23278. doi: 10.1371/journal.pone.0023278. An intriguing result suggesting that some lactobacilli may secrete polyP to exert immunomodulatory effects on the mammalian gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Li Y, Tang CM. The role of the exopolyphosphatase PPX in avoidance by Neisseria meningitidis of complement-mediated killing. J Biol Chem. 2010;285:34259–34268. doi: 10.1074/jbc.M110.154393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray MJ, Wholey W-Y, Jakob U. Bacterial responses to reactive chlorine species. Annu Rev Microbiol. 2013;67:141–160. doi: 10.1146/annurev-micro-102912-142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deborde M, von Gunten U. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: a critical review. Water Res. 2008;42:13–51. doi: 10.1016/j.watres.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 16*.Archibald FS, Fridovich I. Investigations of the state of the manganese in Lactobacillus plantarum. Arch Biochem Biophys. 1982;215:589–596. doi: 10.1016/0003-9861(82)90120-5. The original demonstration that polyP-manganese complexes can functionally replace superoxide dismutase. [DOI] [PubMed] [Google Scholar]
- 17.Culotta VC, Daly MJ. Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid Redox Signal. 2013;19:933–944. doi: 10.1089/ars.2012.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hothorn M, Neumann H, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, et al. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science. 2009;324:513–516. doi: 10.1126/science.1168120. [DOI] [PubMed] [Google Scholar]
- 19.Reddi AR, Jensen LT, Naranuntarat A, Rosenfeld L, Leung E, Shah R, Culotta VC. The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic Biol Med. 2009;46:154–162. doi: 10.1016/j.freeradbiomed.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Barnese K, Gralla EB, Valentine JS, Cabelli DE. Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc Natl Acad Sci U S A. 2012;109:6892–6897. doi: 10.1073/pnas.1203051109. A careful study establishing the mechanism by which managnese-phosphate compounds detoxify superoxide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulaev IS, Vagabov VM, Kulakovskaya TV. The forms in which polyphosphates are present in cells. In: Kulaev IS, Vagabov VM, Kulakovskaya TV, editors. The Biochemistry of Inorganic Polyphosphates. 2. John Wiley & Sons, Ltd; 2004. pp. 45–51. [Google Scholar]
- 22.Richter Y, Fischer B. Nucleotides and inorganic phosphates as potential antioxidants. J Biol Inorg Chem. 2006;11:1063–1074. doi: 10.1007/s00775-006-0143-4. [DOI] [PubMed] [Google Scholar]
- 23*.Rachmilovich-Calis S, Masarwa A, Meyerstein N, Meyerstein D. The effect of pyrophosphate, tripolyphosphate and ATP on the rate of the Fenton reaction. J Inorg Biochem. 2011;105:669–674. doi: 10.1016/j.jinorgbio.2011.01.009. Kinetic studies of the effect of polyP on the Fenton reaction. [DOI] [PubMed] [Google Scholar]
- 24*.Grillo-Puertas M, Schurig-Briccio LA, Rodriguez-Montelongo L, Rintoul MR, Rapisarda VA. Copper tolerance mediated by polyphosphate degradation and low-affinity inorganic phosphate transport system in Escherichia coli. BMC Microbiol. 2014;14:72. doi: 10.1186/1471-2180-14-72. Demonstration that copper tolerance in E. coli depends on polyP synthesis, degradation, and Cu2+/phosphate export via the PitAB transporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remonsellez F, Orell A, Jerez CA. Copper tolerance of the thermoacidophilic archaeon Sulfolobus metallicus: possible role of polyphosphate metabolism. Microbiology. 2006;152:59–66. doi: 10.1099/mic.0.28241-0. [DOI] [PubMed] [Google Scholar]
- 26.Keasling JD. Regulation of intracellular toxic metals and other cations by hydrolysis of polyphosphate. Ann N Y Acad Sci. 1997;829:242–249. doi: 10.1111/j.1749-6632.1997.tb48579.x. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz ON, Alvarez D, Gonzalez-Ruiz G, Torres C. Characterization of mercury bioremediation by transgenic bacteria expressing metallothionein and polyphosphate kinase. BMC Biotechnol. 2011;11:82. doi: 10.1186/1472-6750-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, et al. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 29**.Maisonneuve E, Gerdes K. Molecular Mechanisms Underlying Bacterial Persisters. Cell. 2014;157:539–548. doi: 10.1016/j.cell.2014.02.050. Demonstration that polyP is a critical component of the regulatory circuit leading to formation of persister cells. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Vulic M, Keren I, Lewis K. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother. 2012;56:4922–4926. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maisonneuve E, Castro-Camargo M, Gerdes K. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell. 2013;154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 32.Kuroda A, Murphy H, Cashel M, Kornberg A. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. The Journal of biological chemistry. 1997;272:21240–21243. doi: 10.1074/jbc.272.34.21240. [DOI] [PubMed] [Google Scholar]
- 33.Nomura K, Kato J, Takiguchi N, Ohtake H, Kuroda A. Effects of inorganic polyphosphate on the proteolytic and DNA-binding activities of Lon in Escherichia coli. J Biol Chem. 2004;279:34406–34410. doi: 10.1074/jbc.M404725200. [DOI] [PubMed] [Google Scholar]
- 34.Ault-Riche D, Fraley CD, Tzeng CM, Kornberg A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schurig-Briccio LA, Farias RN, Rintoul MR, Rapisarda VA. Phosphate-enhanced stationary-phase fitness of Escherichia coli is related to inorganic polyphosphate level. J Bacteriol. 2009;191:4478–4481. doi: 10.1128/JB.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiba T, Tsutsumi K, Yano H, Ihara Y, Kameda A, Tanaka K, Takahashi H, Munekata M, Rao NN, Kornberg A. Inorganic polyphosphate and the induction of rpoS expression. Proc Natl Acad Sci U S A. 1997;94:11210–11215. doi: 10.1073/pnas.94.21.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Maghrebi MA, Benov LT. Polyphosphate accumulation and oxidative DNA damage in superoxide dismutase-deficient Escherichia coli. Free Radic Biol Med. 2001;31:1352–1359. doi: 10.1016/s0891-5849(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 38.Maciag A, Peano C, Pietrelli A, Egli T, De Bellis G, Landini P. In vitro transcription profiling of the sigmaS subunit of bacterial RNA polymerase: re-definition of the sigmaS regulon and identification of sigmaS-specific promoter sequence elements. Nucleic Acids Res. 2011;39:5338–5355. doi: 10.1093/nar/gkr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manganelli R, Voskuil MI, Schoolnik GK, Smith I. The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol Microbiol. 2001;41:423–437. doi: 10.1046/j.1365-2958.2001.02525.x. [DOI] [PubMed] [Google Scholar]
- 40**.Sanyal S, Banerjee SK, Banerjee R, Mukhopadhyay J, Kundu M. Polyphosphate kinase 1, a central node in the stress response network of Mycobacterium tuberculosis, connects the two-component systems MprAB and SenX3-RegX3 and the extracytoplasmic function sigma factor, sigma E. Microbiology. 2013;159:2074–2086. doi: 10.1099/mic.0.068452-0. Demonstration of the central position of polyP in the complex regulatory circuit controlling the σE regulon of M. tuberculosis. [DOI] [PubMed] [Google Scholar]
- 41.Sureka K, Dey S, Datta P, Singh AK, Dasgupta A, Rodrigue S, Basu J, Kundu M. Polyphosphate kinase is involved in stress-induced mprAB-sigE-rel signalling in mycobacteria. Mol Microbiol. 2007;65:261–276. doi: 10.1111/j.1365-2958.2007.05814.x. [DOI] [PubMed] [Google Scholar]
- 42.Thayil SM, Morrison N, Schechter N, Rubin H, Karakousis PC. The role of the novel exopolyphosphatase MT0516 in Mycobacterium tuberculosis drug tolerance and persistence. PLoS One. 2011;6:e28076. doi: 10.1371/journal.pone.0028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barik S, Sureka K, Mukherjee P, Basu J, Kundu M. RseA, the SigE specific anti-sigma factor of Mycobacterium tuberculosis, is inactivated by phosphorylation-dependent ClpC1P2 proteolysis. Mol Microbiol. 2010;75:592–606. doi: 10.1111/j.1365-2958.2009.07008.x. [DOI] [PubMed] [Google Scholar]