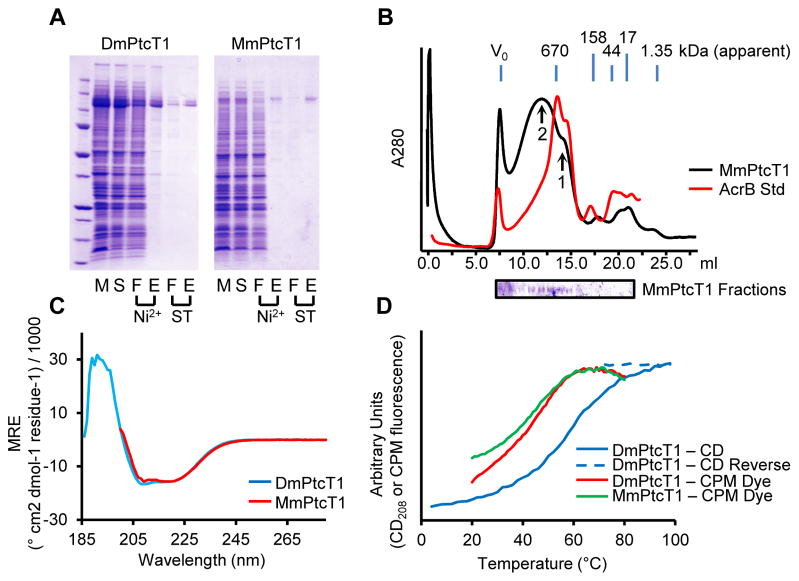
Figure 2. Purification and characterization of Ptc proteins.
(A) DmPtcT1 and MmPtcT1 were purified by successive Ni2+ and Strep-Tactin (ST) chromatography steps. Gel Lanes: (M) resuspended membranes; (S) detergent-solubilized membranes; (F) flow-through; (E) elution (B) SEC of purified, concentrated MmPtcT1 (140 kDa calculated) compared to tagged AcrB (123 kDa), a protein standard with the same predicted topology. MmPtcT1 elutes in two peaks: a small oligomer consistent with a potential physiological trimer (arrow 1) and a larger oligomer (arrow 2) (C) CD spectra of DmPtcT1 and MmPtcT1 show substantial alpha-helical character. (D) Thermal unfolding of Ptc proteins as measured by CD at 208 nm (correlated with alpha helical content) and by CPM dye binding to exposed cysteines.