Figure 2.
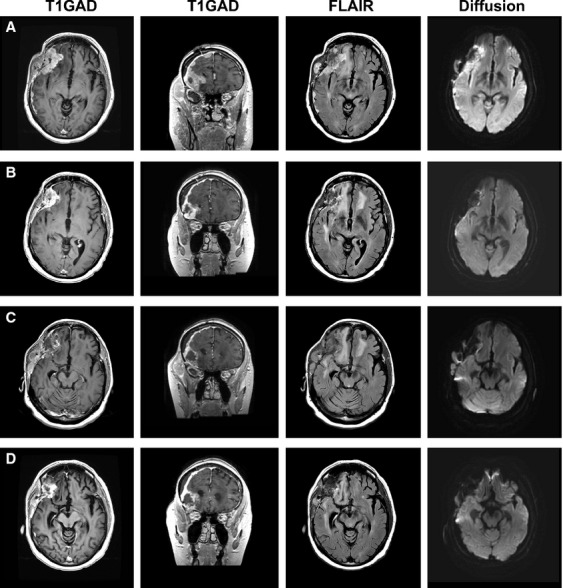
Objective response from NovoTTF-100A, bevacizumab and TCCC. (A) This panel shows baseline size of the recurrent glioblastoma in patient 1 comprising of both intracranial and extracranial components, measuring 6.4 cm × 3.0 cm (19.2 cm2) and 2.7 cm × 1.5 cm (4.1 cm2), respectively. (B) After two cycles of treatment, both intracranial and extracranial components of the tumor shrank to 4.2 cm × 2.4 cm (10.1 cm2) and 2.1 cm × 0.7 cm (1.5 cm2), respectively. There was a reduction of 50% in the bi-dimensional total tumor size, which was derived from a reduction of 47% intracranial and 63% extracranial components. (C) There was regrowth of both intracranial and extracranial components of the tumor 6 months after initiation of therapy. (D) The tumor again had a minor reduction of both intracranial and extracranial components of the tumor upon rechallenge with the addition of 6-thioguanine and lomustine for two cycles.
